REVIEW ARTICLE
Role of Flavonoids against COVID-19
Ambreen Fatima1, Yasir H. Siddique1, *
Article Information
Identifiers and Pagination:
Year: 2021Volume: 9
First Page: 47
Last Page: 55
Publisher ID: TOBIOJ-9-47
DOI: 10.2174/1874196702109010047
Article History:
Received Date: 20/11/2020Revision Received Date: 30/5/2021
Acceptance Date: 02/6/2021
Electronic publication date: 01/12/2021
Collection year: 2021

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The novel coronavirus disease (COVID-19) has entered a threatening stage all over the world. Many lives have been lost, and many more are in need of treatment. The mild symptoms may include fever and dry cough, but in severe cases, it could lead to pneumonia and ultimately death in some instances. Though medical scientists all over the globe are working hard to develop a treatment for this disease, yet no definite cure has been found. To date, the treatment strategy is based on adopting strategies to break the transmission of the virus and repurposing of the old drugs to prevent the loss of life. Among the various potent candidates, flavonoids may play a protective role in these times. Studies have already proven various health-promoting properties of flavonoids in earlier viral diseases, like SARS and MERS. Since ancient times, been plants have used to treat a number of human diseases. Different phytoproducts have been previously described to inhibit the replication of numerous viruses. Despite the positive reports for plant-based medications, no successful clinical trials on phytoproducts as anti-COVID agents have been conducted to date. This review highlights the efficacy of flavonoids as a treatment strategy either alone or in combination with other drugs.
1. INTRODUCTION
The year 2020 observed one of the deadliest pandemics that has threatened the global health, security and economy [1]. It was rapidly shown to be caused by a novel coronavirus, causing severe acute respiratory syndrome [2]. The number of people infected with the severe Acute Respiratory Syndrome (ARD) associated with COVID-19 is rapidly increasing worldwide [3]. The WHO Emergency Committee declared a global health emergency based on growing case notification rates at Chinese and international locations on January 30th, 2020 [4]. The pandemic started in December 2019 in Wuhan, China, and till date, it has covered almost the entire globe. Approximately 210 countries and territories worldwide have been affected by COVID-19 disease (Source:www.stat stica.com). According to the current records of WHO reported on August 23rd, 2020, globally there are 23,057,288 confirmed cases of COVID-19, including a high death toll of 800,906. The country-wise COVID-19 data has been provided in Table 1. In India, the pandemic broke around January 2020, increasing leaps and bounds in the following months. The official website of the government of India reported 710,771 active cases and 57,542 deaths. The data also showed that the cured/discharged individuals accounted for a good 2338035 number, finally surpassing the number of active cases and giving a tiny hope for a brighter and healthier future. Most patients with COVID-19 exhibit mild to moderate symptoms, but approximately 15% progress to severe pneumonia, and about 5% eventually develop ARDS, septic shock and/or multiple organ failure [5]. A number of antiviral drugs, including the nucleotide analogue remdesivir, are being actively tested, but none of them has been approved for the treatment of COVID-19 [5]. Besides the vaccine development, there is also a need for approaches involving natural plant products which can directly target the virus or block viral entry. The present review highlights the efficacy of flavonoids as a treatment strategy either alone or in combination with other drugs against COVID-19.
2. CORONAVIRUSES
Coronaviruses belong to the order Nidovirales, suborder Cornidovirineae and family Coronaviridae [6]. Coronaviruses are a group of RNA viruses that cause diseases in birds and mammals. These viruses lead to respiratory tract infections in humans which may range from mild to lethal. At present, there are seven confirmed human Coronaviruses which are mentio-ned in Table 2. The SARS-CoV-2 (Severe Acute Respiratory Syndrome Related Coronavirus-2) or COVID-19 belongs to the genus Sarbecovirus and is zoonotic in nature. It infects few animal species and also humans [7]. These viruses share around 96.20% homology with bat coronavirus sequences [8].
|
Name |
Cases (cumulative total) |
Cases (newly reported in last 24 hours) |
Deaths (cumulative total) |
Transmission Classification |
| U.S.A | 5,567,217 | 45,960 | 174,246 | Community transmission |
| Brazil | 3,532,330 | 30,355 | 113,358 | Community transmission |
| India | 3,044,940 | 69,239 | 56,706 | Clusters of cases |
| Russian Federation | 956,749 | 4,852 | 16,383 | Clusters of cases |
| South Africa | 607,045 | 3,707 | 12,987 | Community transmission |
| Peru | 576,067 | 9,008 | 27,245 | Community transmission |
| Mexico | 549,734 | 5,928 | 59,610 | Community transmission |
| Colombia | 522,138 | 8,419 | 16,568 | Community transmission |
| Chile | 395,708 | 1,939 | 10,792 | Community transmission |
| Spain | 386,054 | 0 | 28,838 | Clusters of cases |
| Name of Human Coronavirus | Genus | Symptoms | |
| 1. | Human coronavirus NL63 (HCoV-NL63) | alpha-coronavirus | Produce mild respiratory diseases |
| 2. | Human coronavirus 229E (HCoV-229E) | ||
| 3. | Human coronavirus 43 (HCoV-OC43) | beta-coronavirus | |
| 4. | Human coronavirus 1 (HCoV-HKU1) |
||
| 5. | SARS-CoV | Produce severe/lethal respiratory diseases | |
| 6. | SARS-CoV-2 | ||
| 7. | Middle East Respiratory Syndrome Coronavirus (MERS-CoV) |
2.1. Human Coronavirus (COVID-19)
The human coronavirus mainly causes upper respiratory tract problems, including the common cold. The study by Killerby et al. [9] states that these viruses infect most of the people, atleast once or more in the lifetime. Before the present COVID-19 pandemic, two other viruses of the same group, i.e. SARS-CoV and MERS-CoV, also caused a large number of deaths in 2002-03 and 2012, respectively. The COVID-19 has an incubation period of 2 weeks, and the symptoms appear slowly during this period. The virus is known to replicate in the upper and lower respiratory tracts of the patients [10].
2.2. Structure of SARS-CoV-2
The name coronavirus is derived from the Latin word corona meaning “crown” or “wreath”. The name refers to the characteristic appearance of the viruses having fringes or bulbous projections on the surface. The SARS-CoV-2 is a member of the family of largest RNA viruses, and has a genome size of 27-32 kb (125 nm or .125 μm). The COVID-19 structure has a close similarity with that of SARS-CoV. The virus uses ACE-2 (Angiotensin-converting enzyme 2) receptors as the entry point like SARS-CoV. Owing to its similarities with the SARS-CoV, the coronavirus study group of the International Virus Committee on taxonomy of virus termed COVID-19 as SARS-CoV-2. It is a single-strand RNA virus with a positive-sense RNA genome (+ssrna) [11]. COVID-19 virus has basic characteristic structure like the other members of this family. There are 4 important structural proteins in virus, viz. the envelope protein (E), the membrane protein (M), the spike protein (S), and the nucleocapsid protein (N). The N protein is responsible for the proper formation of capsid and the entire structure of the virus, whereas the S protein is important for attaching the virus to the host cell [12, 13]. Out of the 4 proteins, the membrane protein (M) is most abundant. This protein spans three times in the membrane bilayer such that there is a short -NH2 terminal domain outside the virus and a long -COOH domain inside the virus. The spike protein (S) is a type I membrane glycoprotein, and it forms the outer peplomers [11]. The S protein is divided into three subunits: i) ectodomain ii) single-pass transmembrane anchor and iii) short intercellular tail. These structures help in anchoring the viruses to the host cell. The large ectodomain is further divided into 2 subunits, the receptor bending S1 subunit and the membrane fusion S2 subunit. Apart from having the same ACE-2 receptor entry points, both SARS-CoV and COVID-19 have similar types of receptor bonding motifs (RBM) and receptor binding domains [14].
2.3. Proposed Infection Mechanism of the Virus
The study of Zhao et al. [15] proposed that the infection mechanism of SARS-CoV-2 is similar to that of SARS-CoV. The Gln 493 residue of the RBM of SARS-CoV-2 helps in the attachment and fusion of the S protein of the virus to the ACE-2 receptor of the human cells. The ACE-2 proteins, mainly in the lungs, act as a major target of COVID-19. The fusion of the virus to the ACE-2 receptor in lungs causes respiratory problems, which is a significant outcome of COVID-19 infection. Studies have shown that SARS-CoV-2 uses a protein TMPRSS 2 to prime its spike (S) protein. This activation is required for the attachment of S proteins to the ACE-2 receptors. Once the virus enters the host cells, the viral RNA replicates to survive inside the host cell. The viral replication inside the host requires the following main components:
(i) Open Reading Frames (ORF’s): There are 12 ORFs along with a set of 9 sub-genomic mRNAs having a conserved leader sequence, 9 transcription regulatory sequences and 2 terminal untranslated regions.
(ii) 2 replicase genes (rep la and rep lab).
(iii) A 5’-3’ slippery sequence (5’-UUUAAC-3’).
(iv) Polyproteins (pp la and pp l ab).
The first ORF encodes 16 non-structural proteins (Nsp), while the other ORFs encode the structural proteins. The 16 Nsps include Nsp-3 (papain-like protease), Nsp-5 (main protease), Nsp-12 (RNA-dependent RNA polymerase), Nsp-13 (helicase), which are involved in the transcription and replication of the virus. The recent study by Kim et al. [16] showed that Nsp-15 not only plays a part in the replication but also attacks the host immune system during the replication. The final organization of the coronavirus genome is 5’-leader-UTR-replicase-S-E-M-N 3’-UTR-poly (A). Accessory genes are found to be spread within the structural genes at the 3’ end. As stated earlier, the novel SARS-CoV-2 shows a high similarity to SARS-COV, yet it leads to more life-threatening complication than the latter. The difference in transmission and infectivity of the latter may be due to the gain of function mutation in SARS-CoV-2. Studies have shown that COVID-19 has different Nsp-2 and Nsp-3 [17, 18]. The study by Angeletti et al. [19] also showed that Nsp-2 of COVID-19 has an additional mutation, making it more contagious. Moreover, the ORF-8 and ORF-10 proteins in SARS-CoV-2 are also different from SARS-CoV. The study by Coutard et al. [20] showed that the presence of furin-like cleavage site in the S protein of SARS-CoV-2 may be the cause of its increased virulence. The increased transmission rate and infectivity are due to the higher strength of the COVID-19 S protein by which it binds with the ACE-2 receptor.
Like other beta-coronaviruses, the entry of SARS-CoV-2 into the host cell occurs by certain confrontational changes. It is believed that the SARS-CoV-2 utilizes the same mechanism as SARS-CoV to enter the cell. As stated earlier, both have the same entry point, i.e. ACE-2 receptor. When the spike protein binds to the receptor, proteins are relocated cleaving the S protein and activating the S2 domain. This is followed by the insertion of internal fusion peptides into the membrane, resulting in membrane fusion, and thus, the virus enters the cell. Once entered, the ADAM I7 cleaves the ACE-2 into extra membrane space. A study by Li and Clercq [21] showed that a reduction in ACE-2 resulted in alveoli injury and increased the pulmonary vascular permeability. As the virus translates its proteins, the ORF 3a protein activates the NF-kB pathway, leading to the production of pro-IL-iβ [22]. The activation of the ORF 3a also activates the inflammasome complex. The ORF 3a activation also brings about the activation of caspases and the production of ROS. This results in mitochondrial damage and conversion of pro-1L-1B to IL-B forming cytokinesis, thereby causing respiratory distress. The other pathway that may be attacked by the SARS-CoV-2 is the JNK pathway as it is activated by the SARS-CoV-2, and both viruses have analogous proteins.
2.4. Symptoms
According to WHO, COVID-19 affects different people in different ways. Most of the infected people with mild to moderate illness can recover on their own without the need for hospitalization. The symptoms can be divided into the following groups:
(i) Most common symptoms: Fever, dry cough and tiredness.
(ii) Less common symptoms: Aches and pains, sore throat, diarrhea, conjunctivitis, headache, loss of taste/smell and rash on skin or discoloration of fingers or toes.
(iii) Serious symptoms: Difficulty in breathing or shortness of breath, chest pain or pressure, and loss of speech
2.5. Mode of Transmission
According to the guidelines of the Ministry of Health and Family welfare, the Government of India, COVID-19 spreads mainly by the droplets produced by the coughing and sneezing of an infected person. The spread can occur through the following ways:
(i) Direct close contact: The infection can spread by an individual being in close contact with the patient.
(ii) Indirect contact: The droplets from the coughing and sneezing of the infected patient survive for many days on the surfaces they have fallen on. Therefore, coming in contact with an infected surface or cloth and then touching one’s mouth, nose or eyes can transmit the infection.
The major concern for the spread of COVID-19 infection is the asymptomatic patients, as the patients have no clue that they are infected and can be the source of spreading of disease unknowingly.
2.6. Prevention of Spread of COVID-19
The ministry of health and family welfare has suggested the following steps to prevent the spread of the disease:
(i) Social Distancing: Avoidance of gatherings at social and religious places.
(ii) Maintaining a distance of at least 1m between two people.
(iii) Staying home as much as possible.
(iv) Avoidance of physical contact.
(v) Avoiding touching the surfaces.
(vi) Practicing good hygiene: Washing hands regularly with soap and water or cleaning with alcohol-based hand rubs.
(vii) Avoiding touching the face.
(viii) Covering nose and mouth when sneezing and coughing; it is better to cough into your elbow rather than palms.
(ix) No spitting in public places.
(x) Ensuring cleanliness of the surfaces and objects, like doorknobs, handkerchiefs, etc.
2.7. Clinically used Drugs
Every single day, an increase in the number of patients is witnessed yet, till date, no FDA-approved drug is available in the market. The present medication is symptom-based, and organ support is provided in terminally ill cases [23, 24]. The development of drugs against COVID-19 will take time, so the only available option right now is to repurpose the existing drugs in order to reduce the symptoms. Few commonly used drugs to combat COVID-19 are as follows:
2.7.1. Chloroquin or Hydroxychloroquin
It is an anti-malarial drug which has been shown to possess antiviral and immune-modulating properties. This drug acts against COVID-19 by increasing the endosomal pH, and thereby, preventing viral fusion [25]. This drug is used widely in India to combat the infection of SARS-CoV-2. The Health Ministry of India has also given permission for the use of this drug for treating COVID-19.
2.7.2. Remidesivir
It is an antiviral drug used in the treatment of SARS-COV, MERS-COV and Ebola. This drug causes pre-mature termination by entering the nascent viral RNA [26]. A study by Wang et al. [25] showed that remidesivir acted against COVID-19 and blocked viral infection. The Union Health Ministry of India has also allowed the use of remidesivir as a part of “investigational therapy” for emergency uses.
2.7.3. Lopinavir/Ritonavir
These drugs are anti-HIV drugs and fall under the category of medication called protease inhibitors. This drug acts by disrupting proteolytic processing. Studies have shown that the drug in combination with flu drug, oseltamivir, results in complete recovery of patients presenting COVID-19 related pneumonia [27].
2.7.4. Ribavirin
This antiviral drug is used against hepatitis C and respiratory tract viral infections. In a study by Hung et al. [28], a combination of interferon beta1-b, lopinavir-ritonavir and ribavirin reduced the COVID-19 symptoms and shortened the duration of viral shedding. This triple combination was found to be more effective than lopanavir/ritonavir therapy.
2.7.5. Sofosbuvir
This is a drug used against hepatitis C. It acts as an inhibitor of nucleotide polymerase. This is an FDA-approved drug and was last used against the Zika virus [29]. A recent study by Nourian and Khalili [30] showed that sofosbuvir is a potential drug against COVID-19 because of the similarity in the replication mechanism of hepatitis C virus and coronavirus.
Few other pipeline drugs are listed below:
2.7.6. Antiviral Drugs
These medicines target the important life-supporting events of the virus. These drugs inhibit viral replication, ion channels and serine proteases, all of which are vital for the survival of the virus. The earlier viral outbreaks, such as SARS-CoV and MERS-CoV, have been treated with this group of drugs [31].
2.7.7. Anti-HIV Drugs
These drugs are approved by FDA and used against HIV [32]. These drugs are categorized according to their targets, which are reverse transcription, retro-transcription, proteolytic processing, viral cell fusion and incorporation of viral genome into the host.
2.7.8. Anti Inflammatory Drug
Studies have shown that COVID-19 causes a huge inflammatory response in patients. Anti-inflammatory drugs, such as JAK-STAT inhibitors, have the potential to reduce the elevated levels of cytokines and inhibit viral infection. Stebbing et al. [33] showed that the anti-inflammatory drug, baracitinib, when used in combination with remdesivir (an antiviral drug), enhanced the potential of remidesivir to inhibit viral infection.
2.7.9. Monoclonal Antibodies
The S protein of the virus binds to the ACE-2 receptor of the host, thus facilitating the entery of the virus. Targeting these ACE-2 receptors and developing neutralizing antibodies against them might control the severity of the disease [34]. The advantage of this approach is that the host ACE-2 does not change, and thus, there are no chances of any mutation that may hinder the development of the drug [35].
3. FLAVONOIDS
Flavonoids form the largest group of secondary metabolites that are produced by plants. These naturally occurring polyphenols are found universally in all fruits, vegetables and medicinal plants [36]. Recent years have seen an impetus in exploring the potential of flavonoids. From time to time, different studies have shown that flavonoids exhibit an array of health-promoting properties, like antioxidant properties, antiviral properties, anticancer properties, anti-inflammatory properties, and neuroprotective properties [37]. Flavonoids are further divided into 6 subclasses on the basis of the level of oxidation and substitution pattern of the C ring [37]. Different subclasses of flavonoids and their properties are provided in Fig. (1).
The current pandemic has made it very clear that we can never win against the nature. The panic of having no cure against the disease forced the scientists to look up to nature itself to find some remedy against this peril. According to the WHO report, around 80% of the world’s population is dependent on medicinal plants in order to meet their health requirements.
Owing to their abundant health-promoting properties, many plant molecules have been studied and modulated into drugs for a number of diseases [38]. Many plant products have shown promising antiviral properties. A study by Cragg et al. [39] showed that many plants derived formulations can exert their effects on different stages of viral infections. Due to their positive effects and low side effects, many synthetic drugs against viral diseases have been replaced by medicinal plants [40]. Studies have shown that many plants show high anti-viral, anti-inflammatory and antioxidant properties. It may be a very helpful approach to consider plant products for the treatment of COVID-19. Since ancient times, Indian herbs have been used for the treatment of various diseases, including viral infections. The Indian traditional medicinal system is one of the oldest treatment systems in history and plays a vital role in curbing global health problems [41]. A study by Pundari-kakshudh and Kanaki [42] showed that around 25,000 plants have been used in different medicines. A single herb can contain a number of constituents which in combination with the other compounds may produce desirable pharmacological outcomes [43]. The advantage of using herbal medicine for viral infection is that it builds up the immunity by modulating the immune system of the body. The herbal antiviral drugs have also been used in the previous two coronavirus outbreaks, viz. SARS CoV and MERS-CoV. These drugs have also been found to be effective against influenza and the dengue virus. The ministry of AYUSH has listed different medicines under three broad categories, viz. a) preventive and prophylactic b) symptom management of COVID-19 like illnesses, and c) add-on interventions to the conventional care. The complete list of recommended medicinal plants is given in Table 3.
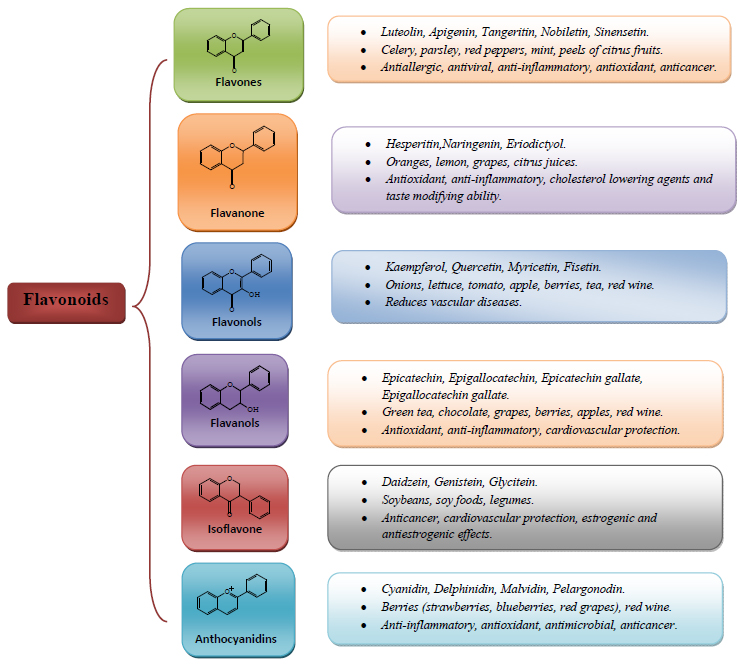 |
Fig. (1). Structure, sources and properties of common flavonoids. |
|
A. Preventive and Prophylactic: 1. Samshamani Vati 500 mg: Twice a day with warm water for 15 days. The medicine contains aqueous extract of Tinospora cordifolia. 2. Nilavembu Kudineer decoction 60 ml: Twice a day for 14 days. The medicine contains aqueous extract of Andrograhis paniculata & others. 3. Preparation of decoction by boiling Behidana (Cydonia oblonga) 3 gm, Unnab (Zizyphus jujube) 5 in number. Sapistan (Cordia myxa) 9 in number in water. 4. Arsenicum album 30: Daily once in empty stomach for three days. |
Effective Against Chronic Fever Effective against cold and fever Have antioxidant, immune-modulatory, anti-allergic and anti-influenza activity Immune-modulator. Effective against SARS-CoV-2 |
Ayurveda Siddha Unani Homeopathy |
|
B. Symptom Management of COVID-19 like Illnesses 1. AYUSH-64 : 02 tablets twice a day 2. Agasthya Hareetaki : 05 gm twice a day with warm water 3. Anuthaila/Sesame oil 02 drops in each nostril daily in the morning 4. Nilavembu Kudineer/Kaba Sura Kudineer— decoction 60 m1 twice a day 5. Adathodai Manapagu — Syrup 10 ml twice a day 6. Arsenicum album, Bryonia alba, Rhus toxico dendron, Belladonna Gelsemium Eupatorium perfoliatum. |
For respiratory infections For respiratory infections For respiratory infections For fever Effective against lung inflammation, viral infections, asthma and lung diseases |
|
|
C. Add on Interventions to the Conventional Care 1. AYUSH-64 : 02 tablets twice a day 2. Agastya Hareetaki: 05 gm twice a day with warm water. 3. Vishasura Kudineer: Decoction 60 m1 twice a day 4. Kaba Sura Kudineer — decoction 60 m1 twice a day |
Effective against fever, cough, sore throat and shortness of breath |
|
Viral infections are difficult to be controlled as the virus is made up of a few building blocks that too are common in all living organisms [44]. This level of similarity between the building blocks of the virus and biological cells makes it difficult to formulate antiviral drugs, as it may hamper the uninfected cells also [45]. Flavonoids act on the virus in a number of ways, as pointed below:
(1) Certain flavonoids inhibit the production of prostaglandins which are needed for the fusion of cell membranes [46].
(2) A number of known viruses, like HIV and poliovirus, rupture lysosome which results in cell damage. The acidification and activation of lysosomes can only be done by their own proton pumps. Flavonoids are known to inhibit these proton pumps, and thus, prevent their rupture and cell death [47].
(3) Flavonoids like quercetin have been proven to inhibit the functioning of the reverse transcriptase of RNA viruses [48].
Pollansky and Lori [49] showed that the use of herbal broad-spectrum antiviral treatment Gene-Eden-VIR/Novirin had a variety of antiviral effects on beta coronaviruses and also on the SARS-CoV. This drug is a combination of various plant products, like quercetin, the extract of cardamom, green tea, cinnamon, licorice and selenium. This herbal concoction was found to be effective against several other viruses also, such as Epstein Bars Virus (EBV), Human Papillomavirus (HPV), Human Cytomegalovirus (hcMV) and also Herpes Simplex Virus (HSV). The individual properties of each component and its possible effect against SARS-CoV are given in Table 4. The above study of Gene Eden Virus/Novirin concluded that the ingredients had a range of antiviral effects, especially on SARS-CoV, which included the inhibition of viral cell entry and infection, prevention of replication, inhibition of viral proteases, amplification of immune response, and decreased production of quasi-species. The study holds importance due to the large-scale similarity of SARS-CoV and SARS-CoV-2. Moreover, to fill in the gap of producing new drugs, these existing drugs can form a potential candidate in the drug development against COVID-19. Citrus flavonoids have also emerged as the promising drug candidate against COVID-19. Utomo and Meiyanto [50], by performing molecular docking, showed the efficacy of compounds present in curcuma species, citrus species, Alpinia galangal and Caesalpinia sappan against SARS-CoV-2. The study used three target proteins, viz. the receptor binding domain of spike protein (RBD-S), ACE-2 (Angiotensin Converting Enzyme 2) of the host and SARS-CoV-2 protease. The study showed that Citrus and galangal compounds showed a higher binding affinity to the receptor proteins. The higher affinity may be the reason for the higher inhibitory effect of these citrus and galangal compounds on viral infections. Out of all the compounds, the citrus flavonoid, hesperidin, was found to be a promising candidate for SARS-CoV-2 drugs as the docking score of hesperidin was observed to be lower than the lopinavir (a presently used drug against SARS-CoV-2). Thus, the present plant products could be suitable prophylactic agents as these are generally consumed through the diet. The study also proves that citrus sp. is the best herb to combat beta coronavirus, including SARS-CoV-2. This study also pointed out that other than hesperidin, other flavonoids in citrus sp. have shown a reduced binding energy for spike glycoprotein and ACE-2 in comparison to lopinavir and nafamostat, respectively. The affinity for these citrus flavonoids of these two proteins will help in inhibiting the attachment of the virus to the host, thus reducing the infection rate. Liu et al. [51] were also successful in crystallizing the main protease (Mpro)/chymotrypsin-like protease (3CL pro) from COVID-19. Using this protease as the main target, a molecular docking study was performed which showed that flavonoids, such as kaempferol, quercitin, luteolin-7-glucoside, naringenin, apigenin-7-glucoside and epicatechin gallate, are the most recommended ones to act as potential inhibitors of COVID-19 main protease (Mpro) [52]. Many earlier studies have also shown the antiviral properties of citrus flavonoids [53-55]. The major advantage of these citrus flavonoids is that they can be either taken as food or mixed with other herbal medicines. Moreover, the peels of the citrus fruits also contain a high content of flavonoids and can also be used to extract these components.
| S.No | Component | Quantity | Property | References |
| 1 | Quercetin | 100 mg | Inhibit both SARS (3CLpro and PL pro) and MERS proteases. Modulates cellular unfolded protein response (UPR) |
[56] [57] |
| 2 | Cinnamon extract | 50 mg | Blocks cell entry via endocytosis. Inhibit wild type SARS infection in vitro. | [58] |
| 3 | Licorice extract | 25 mg | Antiviral and immunostimulating | [59] |
| 4 | Selenium | 100 µg | Supplementation increase immune response to viral infections. | [60] |
CONCLUSION
COVID-19 has emerged as the most frightening viral infection to be handled by mankind to date. Due to the lack of drug against this infection, the governments all over the world have exercised practices, such as social distancing, oral hygiene and quarantine, so as to minimize the human contact. This will hopefully stop the spread of COVID-19 and will also break the transmission chain and prevent the surge in COVID cases. To date, a number of vaccines are available, such as Covishield, AstraZeneca, Moderna, Sinopharm and Covaxin, and it is advised that people should get themselves vaccinated as soon as possible. Most of the vaccines have passed the clinical trials, but there are also reports of several side effects associated with the vaccines. The first mass vaccination program was started in early December 2020. To establish the efficacy and safety of the vaccines, studies with randomized standard controlled design (as well as follow-up) on large samples are required in order to generate confidence among people.
Our review is an attempt to showcase the importance of various plant components that have been used for decades as dietary supplements or as a part of herbal medicines. The undiscovered world of herbal medicine may prove to be a silver lining in these gloomy hours of COVID-19. These plant components, owing to their high antiviral properties, have already been used for treating some viral infections. Some of these components have already shown promising results against SARS-CoV, and owing to the similarity between SARS-CoV and SARS-CoV-2, these components may act against COVID-19 also. Thus, apart from working on potential drugs and vaccinations against COVID-19, due attention should also be given to these natural products that may act together with drugs for positive results.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.




