All published articles of this journal are available on ScienceDirect.
Direct At-Sea Observations of Elephant Seals (Mirounga spp.) to Help Interpret Digital Bio-logging Data
Abstract
Background:
A key short-fall with animal-borne bio-logging instruments, which collect digital time-series data regarding the foraging behaviours of cryptic marine mammal species, is validating those data against in situ behaviours.
Objective:
To collate direct observations of elephant seal feeding behaviour to help interpret foraging behaviours inferred from Time-Depth Recorder (TDR) data.
Methods:
Direct observations of elephant seal foraging behaviour were collated from the published literature using a search of the world-wide-web. Those observations were supplemented with an unpublished record.
Results:
Two deep-sea video recordings and six surface sightings of elephant seals ingesting prey were collated. Each observation either supported or suggested an alternative to behaviours derived from digital time-depth profiles. The tendency for elephant seals to surface following the capture of large prey suggests precipitous drops in stomach temperature at the sea-surface, which have been recorded and interpreted as drinking events, more likely represent the ingestion of large prey items.
Conclusion:
Direct observations of marine mammal foraging behaviours are rare, yet they provide a means to continuously evaluate and interpret outcomes of bio-logging instruments.
1. INTRODUCTION
One way to gather information about highly mobile and migratory species, such as marine mammals, is through the use of bio-logging instruments which, depending on the array of sensors deployed, record aspects of an individuals’ movements and behaviours at set time intervals [1]. However, historical bio-logging studies often lack complementary deployments (e.g. video recorder + TDR), which combine to provide a more comprehensive picture for foraging behaviours that are beyond the boundary of our visibility or experiences [2, 3]. Nevertheless, knowledge of animal migration patterns and foraging behaviours inform us of when, where and how the Earths’ biota interacts with its environment and also with human resource requirements.
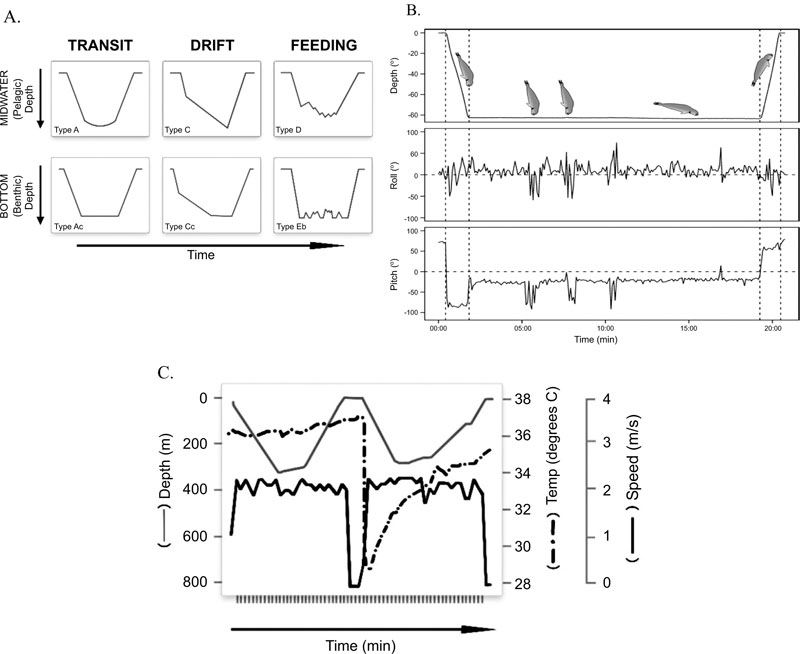
Elephant seals (Mirounga spp.) are large-bodied migratory marine mammals. All age classes have been tracked by satellite telemetry to remote oceanic or continental settings where attached bio-logging instruments have recorded several different functional dive types, including foraging dives where the seals are purported to prey on the suite of benthic and pelagic species during what have been termed “wiggles” in the dive profiles (Fig. 1) [4-7]. Compared with females, male elephant seals are up to 10 times larger and differ in their foraging ecology by preferentially hunting for benthic prey over continental shelves whereas females forage in more open pelagic waters [4]. The cumulative effects of their lengthy temporal migrations to geographically remote locations combined with the near-continuous diving behaviour for both sexes and all age classes [8] makes direct observations of elephant seals a novelty.
Here, we collated direct observations of foraging behaviour for Mirounga spp. We supplement those records with an unpublished image of a male M. leonina ingesting prey at the sea-surface. Our objective is to discuss those results with respect to dive behaviours that have been inferred from two-dimensional data collected using digital TDRs and stomach temperature sensors.
2. METHODS
Reports of direct observations of elephant seal feeding were collated through an internet search using “video footage elephant seal feeding” and “surface feeding elephant seal” as the search terms. Two direct video recordings of elephant seals feeding at extreme depths were found. Two published accounts of elephant seals feeding at the sea-surface were found and those supplemented with a third unpublished observation.
3. RESULTS AND DISCUSSION
3.1. Deep Water Observations
Two studies have serendipitously captured brief observations of feeding in elephant seals. The first was via a video camera anchored to the seafloor at a depth of 894 m in the Barkley Canyon, west of Vancouver Island, Canada. Footage was captured of a free-ranging northern elephant seal (M. angustirostris), of unknown sex, predating a soft-bodied hag-fish (Eptetratus sp., 25/June/2012, https://bmscblog. wordpress.com/2013/04/03/citizen-scientist-spots-a-hungry-elephant-seal-in-barkley-canyon/ (accessed 14/05/2020). No pre/post-ingestion behaviour was witnessed but the seal captured Eptetratus sp. by suction feeding and swallowed it immediately at depth.
The second study captured video footage of M. leonina attacking Patagonian toothfish (Dissostichus eleginoides) hooked on commercial longlines near Heard Island [9] (https://doi.org/10.1371/journal.pone.0172396.s004,and.s005). Although the behaviours recorded were in a somewhat artificial setting (i.e. a lit environment with tethered prey), the footage provides insights into the behaviour of the seals as they encountered benthic prey at depths in excess of 1000 m. Seals swam close to the sea-floor and undertook brief (meters) excursions from the seafloor as they circled hook-caught fish of ~ 1 m in length. Prey capture behaviour included a “head-down” orientation and a turn toward the surface following seizure of the prey item.
3.2. Surface Observations
Three adult male M. angustirostris have been observed consuming large-bodied elasmobranchs such as stingrays (Urolophus halleri), blue sharks (Prionace glauca) and spiny dogfish (Squalus acanthis) head-first at the seas-surface [10]. There was one report of a male M. leonina feeding at the sea-surface on what was believed to be a large fish, perhaps a D. eleginoides, that was presumed captured in about 1000 m of water to the northwest of South Georgia (53º39' S, 38º24' W) [11].
Here, we supplement the record for M. leonina with a further sighting of a sub-adult male (aged 6 - 8 y) swallowing head-first a draughtboard shark (Cephaloscyllium laticeps) in the near-shore surface waters (≤ 70 m) of south-east Tasmania (43º11' S, 147º58' E; Fig. 2). Combined, those five surface observations suggest large hard-bodied prey items captured at depth are bought to the sea-surface for manipulation prior to ingestion.

3.3. Informing TDR Derived Behaviours
What is currently (2020) known of adult male Mirounga foraging behaviours is outweighed by what is known for adult females and juveniles, and for that reason, some male behaviours have been assumed from other age/sex classes. But, behaviours inferred from single-sensor TDR deployments do not always equate with those derived from complementary multi-sensor studies, nor might they be applied across species or even between age classes within species [12-15]. This makes the observations described here valuable for understanding specific foraging behaviours of adult male Mirounga spp.
The behaviours of male M. leonina captured directly by video camera [9] conformed well to behaviours inferred from TDR records. For example, there were small excursions from the seafloor that are characteristic of “wiggle” type anomalies commonly seen in elephant seal feeding dive profiles (Fig. 1A). The head-down orientation observed by video just prior to prey capture has also been digitally recorded and termed “up-ending” in the benthos (Fig. 1B), [16]. It was also clear from the video that not all wiggles and head-down orientations were prey capture attempts, some were searching activities. More- over, up-ending events seem to equate more closely to prey capture attempts than wiggles in feeding dives, confirming the usefulness of complementary sensor deployments.
Observations of Mirounga spp. feeding on large prey items at the sea-surface offer an alternative explanation for the precipitous drops in stomach temperature that have been termed “drinking events” (Fig. 1C), [17]. Practically speaking, stomach temperature telemetry uses measured changes in a predators’ core body temperature to imply consumption of ectothermic prey and/or the ingestion of water [18]. Changes in stomach temperature for one free-ranging female M. angustirostris, whose dive behaviour appeared to be unconstrained by bathymetry, occurred during the bottom phases of putative type D pelagic foraging dives (Fig. 1C) [19]. Feeding was inferred from anomalies in stomach temperature that were associated with increased swim velocity and wiggles (Fig. 1C), and importantly an upward movement through the water column to shallower depths [17]. Unlike other studies [18, 19], Horsburgh and others [17] recorded decreases in stomach temperature at the sea-surface which they assigned to drinking events (Fig. 1C). However, deliberate drinking of seawater in pinnipeds seems equivocal. Harbor seals (Phoca vitulina), for example, did not drink seawater voluntarily [20] but a captive leopard seal (Hydrurga leptonyx) drank large quantities of seawater when placed in the ocean [21].
All Mirounga spp. observed feeding at the surface were manipulating large, hard-bodied, deep-dwelling benthic prey species ([10, 11,] this study) which can reach at least 1.5 m in length [22]. Since the ingestion of large prey causes a rapid decrease in stomach temperature [18], we consider the stomach temperature changes that recorded at the sea-surface by Horsburgh et al. [17] as evidence for the ingestion of large prey. If it is a requirement for Mirounga ssp. to surface to manipulate large prey items prior to ingestion, it follows that anomalously long intervals between dives in the TDR records (known as extended surface intervals [8]) could indicate successful capture of large prey items.
CONCLUSION
Direct observations of feeding by free-ranging, near-continuous deep-diving marine mammals are extremely rare. However, they provide a valuable means for identifying behaviours that will aid in interpreting underwater behaviours digitally recorded with the use of bio-logging instruments [23]. Without verification, the risk of subjectivity and preconceived notions of inferred behaviour will continue, but assigning behavioural activities to digital data remains a challenge [24]. For that reason, continually re-evaluating TDR data free from predilection and by all means available will refine species prey consumption model estimates. Moreover, humans can more completely appreciate the degree to which marine predators overlap with their own requirements for marine resources.
AUTHORS’ CONTRIBUTIONS
JvdH conceived the paper, collated the data and wrote the manuscript.
ST shared the image of a male southern elephant seal feeding in southern Tasmania and contributed to manuscript preparation.
Both authors read and approved the final manuscript.
CONSENT FOR PUBLICATION
Not applicable
FUNDING
Not applicable
CONFLICT OF INTEREST
The authors declare no conflicts of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We thank the reviewers for their constructive comments. The Australian Antarctic divisions’ Dan Broun drew Figs. (1A and 1C).


