REVIEW ARTICLE
A Detailed Biological Approach on Hormonal Imbalance Causing Depression in Critical Periods (Postpartum, Postmenopausal and Perimenopausal Depression) in Adult Women
Nikita Saraswat1, *, Pranay Wal1, Rashmi S. Pal1, Ankita Wal1, Yogendra Pal1, Tamsheel F. Roohi1
Article Information
Identifiers and Pagination:
Year: 2021Volume: 9
First Page: 17
Last Page: 35
Publisher ID: TOBIOJ-9-17
DOI: 10.2174/1874196702109010017
Article History:
Received Date: 18/11/2020Revision Received Date: 15/5/2021
Acceptance Date: 13/5/2021
Electronic publication date: 05/10/2021
Collection year: 2021

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
In today's global world, most women are experiencing midlife health problems which can affect their lives and mental status. Most of the diseases occurr after childbirth and during pregnancy or gestation period which can lead to severe problems such as postpartum depression (PPD), postmenopausal depression, perimenopausal depression which ultimately affects the mental health condition and develop various depressive episodes which ultimately lead to depression in women.
Objective:
The review paper gives the information that if there is untreated maternal depression then it can lead to anxiety, fear, negative effect on child development, disruption of the mother-infant relationship, and the occurrence of depressive symptoms in the early life of infants. Hormone levels are changed at the time of pregnancy.
Methods:
The data was collected by studying combination of research and review papers from different databases like PubMed, Medline, and Web of science by using search keywords like “Postpartum depression”, “Postmenopausal depression”, “Risk factors”, “Pathogenesis of PPD”, “Predictors of postpartum depression”.
Results:
This can lead to disrupting the quality of life of menopausal women like deficiency of nutrients, not properly regular physical activities, elevated Body Mass Index (BMI), loss of libido (loss of interest in sexual activities), due to the lack of education, and awareness among the people. Factors like increase in physical activity can naturally help in PPD condition. Mind body therapy, drug therapy and cognitive and mindfulness-based therapies help in hormonal imbalances.
Conclusion:
It was found that low birth weight and congenital abnormalities in babies lead to affect depression after delivery. It is recommended that health care providers and physicians are provided with information regarding factors contributing PPD and postmenopausal depression. Sleep timings and consumptions of nutraceuticals can help in natural healing with depression amongst women suffering from postpartum, postmenopausal and perimenopausal depression.
1. INTRODUCTION
The maternal feelings of happiness and joy most often evoke from the birth of a child but there is a fact which has minor consideration towards the emergence of Postpartum Depression (PPD), which is also present in new mothers [1]. Major Depression (MD) is the effective mood disorder or neuropsychiatric disorder and chronic illness with a high prevalence rate and is determined as the second largest leading cause of deaths and disability in 2010 in Canada, the United States (US) and globally [2, 3]. One of the most important depressions is the Postpartum Depression (PPD), which is a mood disorder that affects 10 to 15% of newly mothers [4]. In the United States, the prevalence rate of Postpartum Depression (PPD) ranges from 7 to 20%. The risk of PPD in the lifecycle is about 10 to 25%, risk at about two months postpartum is 5.7%, and for six months of postpartum, is 5.6% [5, 6].
2. POSTPARTUM DEPRESSION (PPD)
It is strictly defined in the psychiatric denotation as the condition which indicates a major depressive episode beginning within the first 4 weeks after parturition (childbirth). Most of the women can start showing symptoms later in the postpartum period so that it can also extend to cover the entire first year of postpartum. As compared with major depressive episodes, the PPD symptoms must be manifested for about two weeks, and other symptoms which can notify the presence of PPD in postpartum period are depressed mood, loss of libido and interest in any type of basic activities like sleep cycle, appetite interference, loss of activeness, energy, melancholia (intense sadness), and feeling guilty. Also the occurrence of suicidal attempts and thoughts are present. Because of this, diagnosis of PPD is very difficult because fatigue, changes in sleep cycle and weight variations can lead to changes in the chronic patterns of the body. And these variations makes it challenging to easily observe them in the normal individual [7]. The risk factors played a vital role in the manifestations of PPD such as; low educational level, alcoholism and drug abuse, unawareness among the social or partner supports, low economic and financial problems, and obstetrics factors like unplanned pregnancy, the complications during delivery is the potential risking factors and also other biological and the psychological factors associated with the PPD [8-10]. Because the untreated maternal depression or any other kind of illness can lead to disruption of the mother-infant relationship and can also lead to short- and long-term adverse effects inference to child outcomes [11]. This can lead to negative impacts on children by increasing the risk of impairment of mental and motor activity development, infant cognitive capability, low self-confidence, behaviour retardation or disruption [12, 13]. The better screening or diagnosis of the depression which was occurred after postpartum, is done in primary care and obstetrical care; and if the treatment is inadequate then depression can put women on a track of various affective illnesses and the depression becomes mild, moderate and chronic or having recurrent depressive moods [14].
2.1. Types of Postpartum Depression (PPD)
Types of postpartum affective illness are divided into three categories such as postpartum blues, postpartum psychosis and postpartum depression [15, 16] as mentioned in Table 1. About 300-750 per 1000 mothers globally, suffer from postpartum blues which is resolved from a few days to a week and has some negative impacts whereas postpartum psychosis begins from four weeks postpartum and it can also lead to hospitalization; and this can have prevalence rates ranging from 0.89-2.6 per 1000 childbirths.And in postpartum depression, this can start soon after the childbirth and needs to be treated through various therapies otherwise can lead to disruption of mother-infant relationship, child development and its growth. About 5-7 children of mothers who are suffering from PPD can have the highest cognitive behavioural and related problems as compared with the children of the non-depressed mothers as this can have high prevalence rates as 100-150 per 1000 births [17, 18].
| Depressive Disorders | Prevalence | Onset of Disorder | Duration | Treatments |
|---|---|---|---|---|
| Postpartum Blues | 30-75% | About 3-4 days | Hours to days | No treatment is needed other than counselling. |
| Postpartum Psychosis | 0.1-0.2% | Within 2 weeks | Weeks to months | Hospitalization |
| Postpartum Depression | 10-15% | Within 12 months | Weeks to months | Treatment is usually required |
2.2. Aetiology of Postpartum Depression (PPD)
It remains unpredictable as sometimes it was thought that PPD is caused due to lack of nutrients, such as vitamins, as there are no causative agents that can trigger the PPD or they have not been documented, whereas there are some other studies which tend to depict that the more significant causes of PPD are the changes in a woman’s hormonal level during gestation period (pregnancy) [19]. The levels of hormones that is oestrogen, progesterone, and cortisol (stress hormone) can drop drastically and dramatically within 48 hours after parturition (delivery). Therefore, most of the women are sensitive towards these hormonal changes during the reproductive cycles, especially in menses, pregnancy and menopause [20]. Women who suffered from postpartum depression are sensitive towards these hormonal changes which are dropped dramatically after the delivery of a child [21, 22]. According to some research reports it was depicted that there is a link between the cortisol and the depressive and related disorder symptoms during pregnancy and postpartum stage, while some show that there is no relation between the hormones and the postpartum depression so all mothers can experience these changes, but only 10-15% can suffer from PPD [23].
Changes in hormones during pregnancy and postpartum as mentioned in Table 2 can manifest the postpartum depression because the dramatic changes in the level of hormones lead to the development of anxiety, fear, sadness during puerperium [24]. Several endocrinal changes can lead to neuropsychoendocrinology which can follow a systematic pattern because of a continuous increase in the hormonal plasma concentration throughout the 40 weeks of pregnancy, followed by a dramatic drop in the levels at parturition. Various gonadal steroidal hormones like oestrogen, progesterone, testosterone, Corticotropin-releasing Hormone (CRH), cortisol, oxytocin and many more can adhere to this temporal plasma profile [25-29]. Estradiol increases about 50-folds [30], progesterone 10-fold [31], and prolactin about 7-folds [32]; testosterone can show the moderate increases as compared with the pregnancy periods, and oxytocin levels are increased just before the stage of delivery (parturition). Most of the hormones are suppressed in breastfeeding mothers or women, while prolactin levels change or elevate, and because of this the breastfeeding bouts can trigger the acute increment in both prolactin and oxytocin, while estradiol and progesterone levels are suppressed during lactation amenorrhea [33-35]. The continuous increase of steroid hormones can fluctuate in the negative feedback systems and this is achieved by the feto-placental unit, which ultimately produces large amounts of oestrogen, progesterone and CRH. Apart from this effect on hypothalamic CRH production, cortisol can also have positive feedback on placental CRH production, in the last weeks of pregnancy cortisol produced by the foetal adrenal gland which is the signal derived for the maturation and development of organs of the foetus as well as denoted the time of parturition [36].
| Hormones (Biological Systems) | Pregnancy Stage | Postpartum Stage |
|---|---|---|
| Gonadal steroids |
Estradiol: increases in levels about 50-fold in the third trimester of pregnancy over maximum menstrual cycles. Progesterone: increases in levels about 10-fold in the third trimester |
Oestrogen: Estradiol reaches at the early follicular level at days 1-3 because of slower MCR. Progesterone: follicular levels reached by 3-7 days. Plasma concentrations begin to arise from low follicular levels after 2 weeks, ovulation resumes 4(rare) to 12 weeks postpartum in non-nursing mothers. |
| Androgens | DHEA: MCR and oestrogen levels increase at the time of parturition reflecting maternal stress. | DHEA: levels become normalize. |
| HPA axis |
CBG: increases Cortisol: a marked and steady increase CRH: increases in placental origin ACTH: increases |
CBG: return to normal in about 2 weeks. Cortisol: the decline in levels CRH: rapidly decreases ACTH: rapidly decreases up to 6 weeks of postpartum. |
| Thyroid hormones |
T3: increases T4: increases TSH: increases in levels TBG: 2-3 folds increase |
Thyroglobulin: during 6 weeks there is abnormal production (6 weeks) in 40% postpartum women and 5% develop the thyroid abnormalities and disorders. |
| Polypeptide hormones |
Vasopressin: increases Oxytocin: no change Prolactin: increases |
Oxytocin: increases in lactating (breastfeeding) women Prolactin: returns to after 3 months in non-nursing women. |
| Mineralocorticoids | Aldosterone: increases rapidly | Aldosterone: decreases rapidly |
| Inhibin | Increases | Decreases to normal |
| β-endorphins | Increases | Decreases to a normal state |
| Gonadotropins |
LH and FSH: low hCG: increases |
LH and FSH: remain suppressed for 2 weeks and normal in 3-6 weeks. hCG: decreases in 4 weeks. |
| Abbreviations: MCR= Metabolic Clearance Rate; DHEA= Dihydroepiandosterone; CBG= Cortisol Binding Globulin; CRH= Corticotropin-releasing Hormone; ACTH=Adrenocorticotropin (Corticotropin) Hormone; T3= Triiodothyronine; T4= Tetraiodothyronine (thyroxine); TSH= Thyroid Stimulating Hormone; TBG= Thyroid Binding Globulin; LH= Luteinizing Hormones; FSH= Follicles Stimulating Hormone; hCG= Human chorionic gonadotropin (placental hormone). | ||
2.3. Risk Factors Associated with Postpartum Depression (PPD)
Various risk factors which can cause the postpartum depression (PPD) in new mothers as mentioned in Fig. (1) . Is it a crucial time which is followed by the birth of a baby during which one of the most intense physiologic and psychological changes are taking place in the new mothers?
Most of the women can experience an episode of the onset of depression during the process of pregnancy and the childbirth as it can be depicted as a stressful event of life. There are many psychological stressors identified which are responsible for or act as a risk factor for the initiation of the postpartum affective illness and related disorders [37, 38]. The factors such as marital disharmony, lack of mutual understanding between partners, lack of relationships and life events, have the potential to develop PPD as mentioned in Table 3. As there are more factors like neurobiological interest and a family chart to predict the history of depression [39-41]. According to the clinical evidences stress can act as a risk factor for the development of depression, by using various animal models for screening for the study of PPD by utilizing an exogenous corticosterone or stress-based models. They are used during pregnancy and lactation by which they can easily and sufficiently increase the depression and fear, anxiety-like behaviours and disorders and induce a disruptive and deficient maternal care in rodents [42, 43].
Besides these factors, the impact parenting role of father involvement towards the child was also a risk factor for the emergence of postpartum depression in women. It was found that lower rates of PPD found in women/mothers when the proper high range of satisfaction levels were determined with father/husband involvement. The insecure attachment towards infant was linked with the profound depression in the adolescent's mothers is associated with the negative vibes, thoughts, stress, relationship issues, financial stress problems [44].
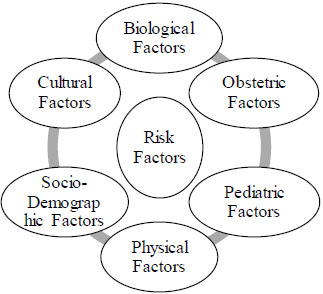 |
Fig. (1). shows the types of factors and life events which are relevant in manifestations of postpartum depression in women. |
| RISK FACTORS OF PPD | Age ˂20 |
| Any substance misuse and abuse | |
| Family history of mental illness | |
| Marriage conflict | |
| Stressful life events like during pregnancy | |
| Hyperemesis, uterine irritability or any psychiatric disorder during pregnancy | |
| Unplanned pregnancy, miscarriage | |
| Lack of emotional and financial support from partner | |
| A congenital malformed infant | |
| Personality and bipolar disorders | |
| Poor relationship with own’s mothers as well as no breastfeeding |
Today women who experienced the occurrence of the multiple adverse life events, including sexual abuse, childhood sexual abuses and adult sexual abuse, were found to be more prone to have increased risk of postpartum depression. These conditions or factors can cause changes in the physiological behaviour of the women and cause disruption of the mental level. Nowadays women who are suffered from the postpartum depression, had 3-5 times higher Everyday Stressor Index (ESI) ranges as compared with the healthy women who are not suffered from PPD and relevant psychiatric mental disorders [45].
These all kinds of numerous environmental risk factors conclude the identification of the postpartum depression and include various depressions like prenatal depression, prenatal anxiety, disruption or impaired infant-mother relationship, lack of confidence and social supports, financial and marital status stress and many other adverse life events [46-48]. The risk factors can act as triggering agents for depression and it is more prevalent in women than men because of internalizing and externalizing of each symptom [49]. In a research study of dizygotic twins, women can show more sensitivity to the interpersonal relationships whereas men show more sensitivity towards factors like external career, goal and future perspectives orientation [50]. Due to the changes in the ovarian hormones, it could also contribute to a high prevalence rate of depressive illness and associated disorders including, premenstrual dysphoric disorder, postpartum depression and postmenopausal depression and anxiety in women. However the mechanisms remain uncleared and it treatments specific to women have not been developed. And the society derived risk factors for depression in women are more likely to have biological aspects, such as the differences in the physical strength and personality traits and energy levels, which can also lead to a higher prevalence of depression in women [51].
2.4. Symptoms of Postpartum Depression (PPD) and Key Signs
The symptoms differ from the non-postpartum depression, as most of the women who are suffering from postpartum depression (PPD) have no psychiatric or mental illness history and they are resistant to symptoms [52]. The clinical manifestations or emerging symptoms of the postpartum depression include sleep cycle disturbances, excessive thoughts, crying, mood swings, change in mental and behavioural patterns, difficulty in thinking, remembering and concentrating, feeling of sadness, doubt, guilt, fear, change in appetite, suicidal thoughts, melancholia, and excessive care of baby and many more as mentioned in Table 4 [53-55].
Many researchers and experts in the field of science consider that postpartum depression shows symptoms and extend to 12 months after delivery [56]. About 60% of women with PPD are diagnosed with obsessive thoughts, focussing, and aggression to their infant as they do not show a desire to hurt the infant most of the time or overcaring leading to avoidance of the baby to minimize these thoughts [57]. Most of the genetic studies determine that depressive episodes begin within the first four weeks of parturition (delivery of the child) [58].
| SYMPTOMS OF PPD | Agitation |
| Risk of infanticide and suicidal attempts and thoughts | |
| Anxiety, fear, crying spells, melancholia, sadness | |
| Inability to sleep or sleeping a lot when the baby is awake | |
| Fear of harming and change in appetite | |
| Extreme concern about the baby | |
| Difficulty in remembering and focussing | |
| Loss of interest in any kind of activities | |
| Paranoia, hallucinations, delusions | |
| Recurrent thoughts of suicide and planning also |
2.5. Predictors of Postpartum Depression (PPD)
There are various types of predictors which can predict the pathogenesis or pathophysiological conditions and mechanisms of the Postpartum Depression (PPD) as shown in Fig. (2). There are various biological and physiological changes which characterize normal human pregnancy because these biological changes are designed to maintain the pregnancy, trigger labour, support the foetal development and maturation, parturition, and lactation. The female body physiology can have the potential adaptive capacity. After the delivery of the baby and, the placenta maintains the maternal-placental-foetal unit throughout the gestation period which was sustained by intricate balance. Due to this, the maternal system undergoes various types of biological changes drastically within the first postnatal days and these adjustments and fluctuations lead to an impact also on maternal health. And the biological predictors can emphasise on the endocrine systems, immune/inflammatory factors, and genetic factors which ultimately cause postpartum depression [59]. On the other hand, psychosocial predictors lead research towards the PPD as it will have a longer period history as compared with biological research factors. They depend on two factors that are stress and interpersonal facts. During pregnancy and delivery, there is an abrupt change occurring in the physiology of the mother and thousands of sudden changes in a woman’s roles for responsibilities and caring and demands towards the new baby. In the past few decades, there has been stress on showing perspective for PPD development because it is paired with other social, psychological, biological, and interpersonal vulnerabilities [60, 61] as mentioned in Table 5.
This stress can lead to the destruction of the mother-infant relationship and caring [62] and harms pregnancy [63]. These psychological and psychosocial predictors act as biomarkers for postpartum depression which was documented only in cases of poor mental health conditions in developed countries, worst life conditions [64], history of premenstrual dysphoric disorders, and stress [65]. These factors associated with PPD and psychological abuse were reported in developed countries [66].
Circadian rhythm has played a vital role because of any dysregulation or alteration in mood changes due to the dramatical variations in the sleep patterns, lack of sleep in the first months of postpartum, and struggling with the issues of life which have been associated with both new-onset and recurrence of PPD [67, 68]. By using these Postpartum Depression Predictors Inventory-Revised (PDPI-R) shows the distribution of risk factors for PPD at different times. Depression and antenatal depression are associated with PPD at six months postpartum. Antenatal anxiety occurs during the first trimester of pregnancy and it is not a predicted postpartum depression but is determined during the last phase of pregnancy. And during this period, the lack of social and socio-economic support and status can predict the occurrence of postpartum depression [69, 70].
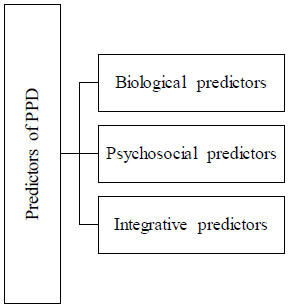 |
Fig. (2). Shows different types of predictors which have the potential for the occurrence of the postpartum depression in women and leading to worsening conditions. |
Table 5. Different types of predictor and related studies and systems which are responsible for PPD in women. [71-80]
| Mechanisms of the Predictors which show PPD | References | |
|---|---|---|
| Biological Predictors |
Endocrine system mechanism • Oxytocin: Lower levels of this hormone lead to PPD because women are suffering from a decrease in gonadal steroids. • Prolactin: less breastfeeding and changes in maternal behaviours in PPD women. • Oestrogen: due to increased sensitivity to oestrogen signalling causes changes in oestrogen-sensitive transcript gene expression which produces an elevation in mood. As these changes lead to disrupting the HPA-axis (hypothalamopituitary axis) and decrease the levels of hormones. • Stress hormones (cortisol, adrenocorticotropic hormone): the dysfunction and disruption in hypothalamopituitary axis (HPA) lead to an elevation in hormones level and manifest PPD and chronic stress paradigms. • Neurosteroids: fluctuations in the levels of allopregnanolone show PPD during late pregnancy and genetic polymorphism in neurosteroids. • Immune/inflammatory responses: Excessive activation of inflammatory responses is involved in the pathogenesis of PPD because excessive activation of pro-inflammatory immune responses causes stress and tissue damage. • Genetic and epigenetic studies: polymorphism in serotonin receptor, dopamine and methylation and alteration in DNA implicates PPD. |
[71] [72] [73, 74] [75] [76] [77, 78] [79] |
| Psychosocial Predictors | • Stress and marital relationship status can lead to PPD in most of the women who suffer from various types of adverse stress life events. | [80] |
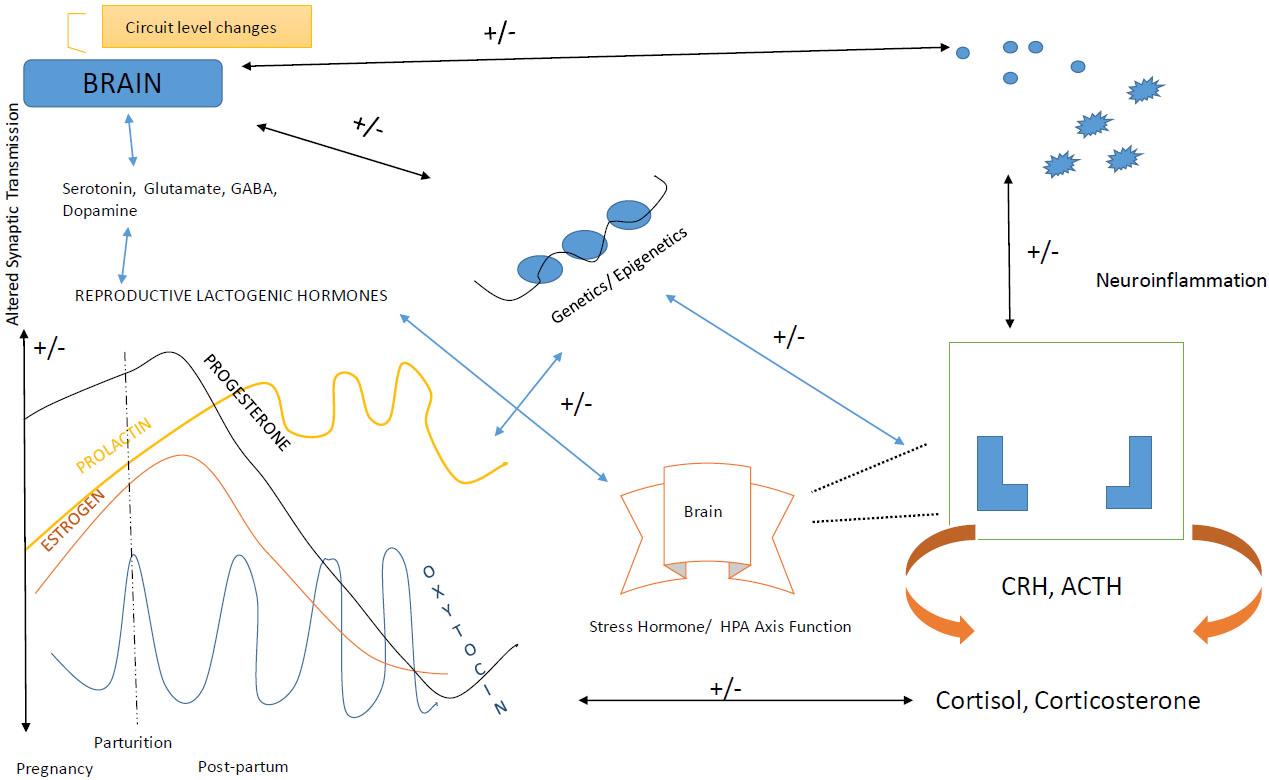
2.6. Epigenetic Mechanisms, Circuit Mechanisms in Postpartum Depression (PPD)
2.6.1. Epigenetic Mechanisms
The inheritance of PPD is done by basic genes and the pathways which if associated with risk factors mentioned above, leads to the occurrence of symptoms during the postpartum period of the women. The methylation process or the modifications in the histone proteins is held by epigenetic factors which change the structure of the chromosomes (gene expression) and in turn trigger unrelated changes in DNA nucleotides sequences. These changes are held to manipulate the transcription factor which has the key role in any mechanism held in the body [67]. The epigenetic factors are the factors of the relationship between the environmental influences and genetics community. Heterochromatin protein 1, binding protein 3 (HP1BP3) and the tetratricopeptide repeat domain 9B (TTC9B) are the genes which have the capability of synaptic plasticity and also have oestrogen signalling factors [68]. The expression levels are held in between these two genes and with epigenetic modifications lead to known as a biomarker for the prediction of PPD who are at risk. The regulation of OXTR genes due to this factor and hormonal fluctuations causes different variations in DNA methylation of the particular OXTR gene in a woman who is suffered from PPD. The interaction among the gene and epigenesis causes changes in neuroendocrine mechanisms and the negative correlation between estradiol levels and the ratio of allopregnanolone to progesterone hormone, and affects biochemical pathways with PPD [69, 70] (Fig. 3).
2.6.2. Circuit Levels Mechanisms
Various circuit levels participated in the “maternal care network” which influence the mother and infant relationship during pregnancy after childbirth. The alterations in specific network circuits which are associated with PPD include altered white matter connectivity of the brain, i.e. neuronal oscillations chains in women. The different imaging techniques such as molecular level imaging, through Magnetic Resonance Imaging (MRI), the studies of images of functional activity which stimulate in response to infant-mother relationship stimuli, involve brain parts such as the amygdala, prefrontal cortex, striatum, insula (present in normal maternal care towards her child). And disruption occurred in these regions manifests the occurrence of PPD in women and played a critical role in producing symptoms like suicidal attempts, no likeliness towards her child, disruption of infant-mother relationship, no motivation towards life, and health behaviour patterns damage [70].
There were various shreds of evidence from the researchers who proved that postpartum depression leads to the functional changes in the women body and it is not related to structural changes, and due to this it is known as “state-dependent disorder”. And if any potential disruption found in these regions, they were involved in the pathogenesis of postpartum depression in the mid-aged life of women.
3. DIAGNOSIS OF POSTPARTUM DEPRESSION (PPD)
Diagnosis is similar to that of depression because of numerous physiologic and pathologic conditions. Physical examination of women is necessary and include those who are suffered from postpartum depression and/or other postpartum affective illnesses such as postpartum blues, which was basically occurred in 50-60% of mothers following delivery, peaks around the 4th day after parturition and resolves by the 10th day followed by the examination of the the symptoms like anxiety, fear, lack of sleep, confusion, brief crying irritation [81-85]. Postpartum psychosis manifests bipolar disorder and acts as a diagnostic possibility in women during the puerperal period (higher risk of mood change) and its estimated range is 33-50%, its peaks in the first two weeks after delivery and common in first-time mothers about 35 years of age and older [86, 87]. Diagnosis is also held by proper assessment of thyroid function because hypothyroidism and hyperthyroidism can elevate in the mood behaviour. Thyrotoxicosis can produce symptoms of panic disorder, occurs in 4-7% of patients, elevates after 4-6 months postpartum, and can show problems which produce postpartum depression [88].
3.1. Treatments of Postpartum Depression (PPD)
Before giving treatment, therapies or medications, there has been increasing focus on the early detection of depression after or during pregnancy. Screening of PPD was performed in a 4-6-week postpartum period or the 2-month well-child visit [89, 90]. The most common method for the screening of PPD is the Edinburgh Postnatal Depression Scale (EPDS) as this screening tool is based on a simple 10-item questionnaire which gives the information on suicidal ideation initially proposed by Cox et al. in 1987. Another screening tools are the Postpartum Depression Screening Scale (PDSS) and Physicians' Health Questionnaire (PHQ-9) used to detect PPD [91-93]. Edinburg Postpartum Depression Scale was the most widely used tool for estimating PPD in studies for research purposes. It was found that low birth weight and congenital abnormalities in babies have an effect on depression after the delivery. It is recommended that health care providers and physicians should be provided with information regarding factors contributing PPD and postmenopausal depression.
Most researchers, scientist and scholarly journal articles support that postpartum depression is treatable by knowing the root cause of problem and treatment can be both pharmacological and non-pharmacological. There are two therapies, first-line therapy and second-line therapy. First-line therapy is considered with Psychotherapy in which mothers prefer psychological treatment. The most commonly used therapies under psychotherapy and found to be beneficial are interpersonal therapy (IPT) and cognitive behavioural therapy (CBT) [94]. In IPT, the patient and physician select one of four interpersonal problems like dispute, grief, lack of understanding) etc. as an ailment focus. During therapy which lasts for about 12-20 weeks, patients are assisted in problematic approaches and conditions to build up the relationship with better social support. IP addresses the relevant areas of PPD conditions such as bonding between mother-infant relationship, mother and partner mutual relationship, etc. [95]. Cognitive Behaviour Therapy (CBT) is based on perceptions and behaviours which are ultimately linked with the mood. CBT focusses on caring the patients who are depressed and modifies the negative impacts or thoughts and enhances to makes behavioural changes which lead to generating capability and potential to reduce distress and coping with the social factors. There were many trials held to assess the CBT or other interventions for the treatment of PPD [96]. A clinical trial conducted on 120 women who recently gave birth to a baby, showed that interpersonal psychotherapy was effective towards the ailment of depressive episodes and treated the symptoms and also used for improving the psychosocial functions in the PPD treated women as compared with the control groups who were on the list of such therapies [97]. Whereas the second-line therapy is pharmacotherapy in which a Selective Serotonin Reuptake Inhibitor (SSRI) acts as the first agent because it is associated with low toxicity if patients are taking an overdose, and there is ease of administration. Randomized controlled trials are held and conducted over 87 women who are suffered from PPD compared with fluoxetine, placebo and counselling (cognitive behavioural therapy). These subjects were divided and randomized into one of four groups who received either fluoxetine drug or placebo plus the addition of one or six CBT sessions [98]. Fluoxetine has shown better efficacy as compared with placebo and reduced the symptoms of depression and also showed improvement. Women who breastfeed are excluded from this trial because all the antidepressant medications are excreted in breast-milk and have potential to transfer the effects to the child due to breastfeeding [99]. There was a pooled analysis of Selective Serotonin Reuptake Inhibitors (SSRI) which found that sertraline, paroxetine, and nortriptyline are the least detected in infants and with rare side effects. Also detectable levels of fluoxetine and citalopram are found in infant serum [100-102]. Most of the physicians prescribe the antidepressant medication as mentioned in Table 6 before prescribing any new treatment because if the patient has positive responses towards certain and specific drugs, then that agent will be considered as the first choice for the ailment of the disorder [103].
As there is no evidence which can suggest that one antidepressant drug has the potential for treating women who are suffered from postpartum depression who are not breastfeeding, the choice of medication should be based on patient's history and tolerability profile [ 104 ] .
3.2. Non-Pharmacological Treatments of Postpartum Depression (PPD)
Other non-pharmacological treatments are used for the treatment of postpartum depression (PPD) are Electroconvulsive Therapy (ECT), Bright Light Therapy, Omega-3 fatty acids, Acupuncture, Massage and Physical exercise.
3.2.1. Electroconvulsive Therapy (ECT)
Is an option for the treatment of postpartum depression in postpartum women who are non-responding to the antidepressant medications and have severe psychotic conditions and symptoms; and this study was conducted on small scale of about 5 women and reported 100% success rate [105].
Table 6. Shows the antidepressant medication for postpartum depression (PPD) in women and also shows the adverse effects. [103,104].
| Antidepressant Medication for PPD | |||||
|---|---|---|---|---|---|
| Drugs | Initial Dosage | Treatment Dosage | Maximal Dosage | Adverse Effects | Excreted in Breast Milk |
|
Selective serotonin reuptake inhibitors (SSRI) Citalopram (Celexa) Fluoxetine (Prozac) Sertraline (Zoloft) Paroxetine (Paxil) |
10 mg 10 mg 25 mg 10 mg |
20-40 mg 20-40 mg 50-100 mg 20-40 mg |
60 mg 80 mg 200 mg 50 mg |
Headache, nausea, diarrhoea, sedation, insomnia, loss of libido, tremor, nervousness and delayed orgasm | More excreted in milk. Less excreted in milk |
|
Serotonin-norepinephrine reuptake inhibitors Venlafaxine, extended-release (Effexor XR) |
37.5 mg | 75-300 mg | 300mg | Headache, nausea, sedation, insomnia, sustained hypertension | - |
|
Another antidepressants medication Bupropion, extended-release (Wellbutrin XL) |
150 mg | 150-300 mg | 450 mg | Seizures (0.4%), dry mouth, agitation, nausea, sweating | - |
| As there is no evidence which can suggest that one antidepressant drug has the potential for treating women who are suffered from postpartum depression and are not breastfeeding, the choice of medication should be based on patient's history and tolerability profile [99]. | |||||
3.2.2. Bright Light Therapy
Was introduced for the treatment of seasonal affective disorder and it gives an attractive option for the treatment of perinatal depression, because it does not have any known risks to the foetus or nursing infants [106].
3.2.3. Physical Exercise
Played a vital role in the treatment of PPD because it is defined as “feasible and effective” and have the intensity to perform activities for at least 30 minutes per day, five days a week, in the form of “pram pushing” [107].
3.2.4. Omega-3 Fatty Acids
Are used in the treatment of perinatal depression because they show more benefits to the health of pregnant and postpartum women as well as show their potential effects which positively affect the mood in the general population. Omega-3 fatty acids such as Eicosapentaenoic Acid (EPA) and docosahexaenoic acid (DHA)which are found in fish oils and act as building nutrients for the development of the Central Nervous System (CNS) of the baby in uterus and also help in depletion of maternal omega-3 fatty acids during the gestation period which facilitates the depression process [108, 109].
4. PERIMENOPAUSAL DEPRESSION
The female reproductive life cycle maintains all the events which were necessary for its normal menstrual cycles, pregnancy, gestation period, peripartum period, postpartum period, menopause, post-menopausal and sexual relationships. The perimenopause is the phase of the female reproductive life cycle which describes the phase of transition from the regularity of menstruation to its permanent cessation (amenorrhoea) condition that is from reproductive to non-reproductive life events. And this phase is considered as the critical period for the mid-aged women because of loss of underlying ovarian follicular activities and is undergoing various social, physical, and emotional changes; and it is determined by various biological, environmental, socio-cultural/economic, marital effects, and clinical changes during the ageing process. The prevalence rates are very much high during this period and that changes fluctuate the physiological system of body changes in the certain regions of the brain and manipulate the neuroendocrine mechanisms either by environmental or lifestyle socio factors and lead to the occurrence of Perimenopausal depression before permanent menopause condition [110, 111].
In this transition phase, many women show psychological, somatic, vasomotor and urogenital symptoms. This type of depression has a wide range of symptoms and it is considered as typical depression which both men and women both experience. About 70% of the women have symptoms such as hot flushes, night sweats, sleep disturbances, lethargy, fatigue, irritability, loss of memory/thinking, isolation, loss of libido, hostility and decreased self-respect. In respect to these physical symptoms, the menopausal transition phase acts as a critical period for the occurrence of various psychological depressive episodes and disorders such as depression, anxiety and suicidal attempts. As such the depressive symptoms which were shown are not only dependent upon the reproductive status but also on the past depressive disorders/symptoms occurred in earlier life showing a higher risk of recurrence of the depressive symptoms in perimenopause period. Despite this even mentally healthy women also experience negative mood through depression during this phase. As such the symptoms of perimenopausal depression fluctuate in worsening the condition to severity due to which the diagnostic tools were used for identification. The cognitive symptoms including paranoia, irritation, negative suicidal thoughts, unhealthy behaviour are present in perimenopausal depression; and comparison between major depressive disorders found that the symptoms are same for both [112]. So, these women need to adjust their transition period phase and consider a high quality of life and their well-being and ignore all the resilient factors which are capable to produce negative thought of the perimenopause. Here, resilience is the term which is determined by the capability of the person to successfully withstand, adapt or cope with the symptoms of depression, and recover him/her from significant stress and the adversity of variations, as shown in Fig. (4) [113].
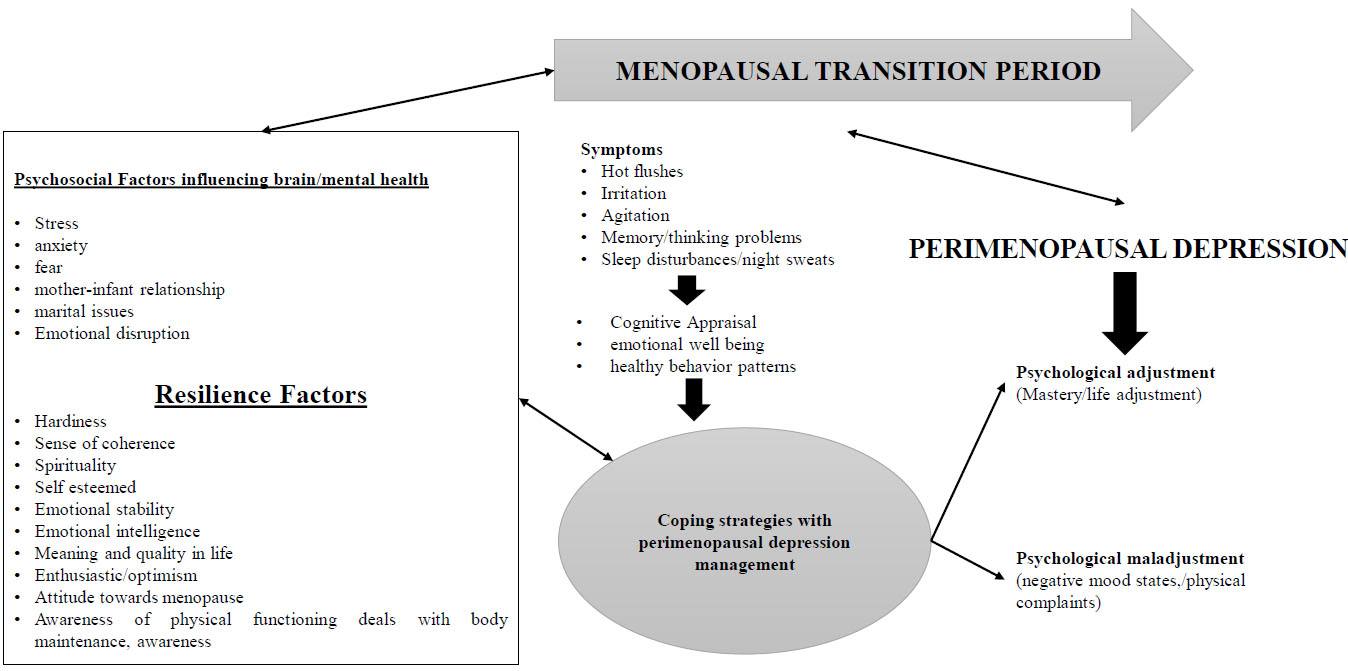 |
Fig. (4). Showing resilience factors coped with symptoms of perimenopausal depression. |
The perimenopausal transition period is of three stages which are divided based on the endocrine (ovarian and hormonal progressive functions), menstrual cycle, stages of reproduction, and ageing, and are as follows:
- Early Menopausal Transition (EMT): change in length of the menstrual cycle which increases about more than 7 days in a woman.
- Late Menopausal Transition (LMT): change and skipping of the 2 or more menstrual cycles and have 1 intermenstrual in about 60 days or more.
- Early Postmenopausal Transition (EPT): women who do not experience the menstruation within the past 12 months [114].
It is important to investigate the proper physique and physical health of a woman who is prone to perimenopausal depression and associated symptoms with other disorders such as thyroid, autoimmune disorders. Due to this, a good physical baseline is produced for menopausal changes and hormonal treatments. The treatment of the perimenopausal depression is done either by biological/pharmacological or psychosocial treatments and they needed a better biopsychosocial approach for a better therapy.
4.1. Psychosocial Treatment
Psychotherapy, useful interventional therapies, general well-being which includes yoga mindfulness-based therapies, change lifestyle factors such as less alcohol intake.
4.2. Pharmacological Treatment
Synthetic steroid such as Tibolone with a hormone makeup induces minor intermenstrual bleeding and treats perimenopausal depression. Bioidentical hormones are those compounds /drugs which resemble the ovarian hormones (oestrogen/ progesterone), but they are used in a limited manner and not recommended by the International Menopause Society. Antidepressant drugs were used such as SSRI (first-line drugs), SNRI (second-line drugs). However, these two classes of drugs have adverse drug reaction like agitating, irritation, sedation, with perimenopausal insomnia, anxiety, leading to worsening the symptoms with drugs such as fluoxetine. Combining hormone therapy with antidepressant drugs is used for the treatment of perimenopausal depression followed by a decrease in the depressive symptoms. As a single therapy is not responding to either treatment alone,so this combined treatment needs to be monitored carefully [115-117].
The rising high levels of the Follicular Stimulating Hormone (FSH) is the very first hormonal change which is caused by reduced levels of gonadotropin hormones which are secreted from ovarian follicles as menopause reaches. Oestrogen levels increase at the time of follicular growth and pituitary gland of brain stimulate the secretion of FSH (to inhibit the growth and maturation of other follicles cells) and Luteinizing Hormone (LH); as these two hormones have negative feedback mechanism towards the ovarian gonadal sexual hormones such as oestrogens and progesterone in females. During the menopausal transition period, these gonadal sex steroids are circulating and fluctuating and later produce granular cells of the ovaries due to reduced levels of oestrogens and inhibin. However, the serum levels of FSH and estradiol, oestrone (oestrogens) are high in the perimenopausal stage and vary from woman to woman and cycle to cycle. But the confirmation diagnosis is done by laboratory testing and depends upon woman who reports about her past and early period. And about after 1 year of perimenopause, the women reach to postmenopause in which woman experience permanent cessation of menstrual periods (amenorrhoea condition) due to low oestrogen and progesterone hormone level and with a high level of gonadotropins [118].
Causal Mechanisms of perimenopausal depression is related to a different hypothesis which suggests why most of the women would be prone to experience depression during perimenopause. Al these symptoms are experienced by 45-85% of women, hot flushes (perspiration during sleep) mechanisms are not completely determined but there is a hypothesis regarding their mechanism of action which suggests that change in the thermoregulatory system in the hypothalamus region of the brain may lead to elevate the levels of sex steroids (gonadal) and the opioid peptides. So, most researchers /clinicians suggest that oestrogen administration decreases the occurrences of hot flushes depend upon dose-dependent fashion [119].
5. POSTMENOPAUSAL DEPRESSION
In this review paper, we also focus on another type of depression which occurs in most of the women after menopause that is Postmenopausal depression. Menopause is unlike a disorder or disease, which is a natural process that all women go through in their lives. This transition and occurrence are accompanied by a number of the common presence of physical and emotional symptoms which include vasomotor symptoms [120, 121], sleep disturbances, difficulties, [122] urogenital problems and sexual dysfunction [123], anxiety and ultimately depression [124, 125]. It is estimated that most of the women about 75-85% suffered from these symptoms, which can lead to cessation of the menses, also known as perimenopause, through into postmenopause [126]. As the menopausal stage experience involves multiple interactions between the psychological, social, cultural and environmental factors, so different types of biological changes are related to alteration in the ovarian hormone status and its deficiency [127, 128]. Most of the researchers published articles which are based on the assumptions that alteration and fluctuations in the sex hormones in the female reproductive system can affect the neurochemical pathways which are related to depression [129, 130].
5.1. Risk Factors Associated with Postmenopausal Depression
Women are particularly susceptible to mood and behaviour disorders during stages of hormone changes and this is explained by the interactions among the neuro-modulating effects of oestrogen on the serotonergic system [131]. These changes in the reproductive events lead to dysregulation of the serotonergic and noradrenergic systems which are involved in mood and behaviour. There is a hypothesis that a reduced capacity to adapt fluctuations and alterations of estradiol or progesterone are predisposed in certain women and lead to depression [132, 133]. In addition to these factors, lifestyle factors also played a vital role in postmenopausal depression such as smoking, other medical conditions, ethnicity, age factors, and employment status. All these factors are associated with increased risk of depression and depressive episodes during menopause [134-136]. Many studies conducted show various factors which are responsible for disturbing and deteriorating the quality of life in the menopausal women [137]. The factors which are responsible for postmenopausal depression, are shown in Table 7, and other depressive disorders that are associated with the early and late menopause in females interact with age factors and increase the risk of the onset of several various chronic disorders and diseases such as cardiovascular diseases, breast and endometrial cancers and osteoporosis [138].
| RISK FACTORS OF POSTMENOPAUSAL DEPRESSION | Frequent Vasomotor Symptoms (Hot Flashes, Sweating) |
|---|---|
| Social lifestyle and status | |
| Stressful life event, Lifetime history of anxiety disorder | |
| Tobacco/ smoking | |
| Elevated Body Mass Index (BMI) | |
| Cultural factors | |
| Lack of physical activity | |
| Employment status | |
| Neuronal fluctuations |
The aetiology of the perimenopausal depression, oestrogen alteration, fluctuation and loss act as: a predisposing factor, when they increase its limbic vulnerability to environmental stressors; act as a precipitating factor when they trigger the gene expression and cause manipulation in genetic field and produce major depression; act as a maintenance factor, when there is a lack of hormonal therapy; thus these factors can worsen the conditions either it is neuronal or genetic.
5.2. Predictors of Postmenopausal Depression
There are various independent predictors of menopausal depression which are frequent episodes of depression in reproductive age, premenstrual syndrome, postpartum depression, and comorbid symptoms like hot flashes, insomnia; elevation in the BMI (Body Mass Index); social and cultural status [139]. Moreover, women who are experiencing bilateral oophorectomy without oestrogen replacement are more affected from major depression [140, 141]. The chronic deficiency of oestrogen hormone can produce a negative impact on the brain major systems: the neurovegetative [142, 143], the emotional/effective [144], and the cognitive and motor [145-147]. The symptoms which were noticed in women who are suffering from menopausal depression after postpartum according to the type of neuronal damage, can produce are shown in Table 8.
As menopause is the influential period during which women undergo a new biological transition, so women with postmenopausal depression have lower levels of estradiol and serotonin concentrations, in spite of high levels of Follicle Stimulating Hormone (FSH). These hormones are having trophic effect on brain, disrupting the nervous system and producing various depressive affective disorders in the mid-aged pregnant women [148].
5.3. Treatments for Postmenopausal Depression
There are various therapies and treatments for postmenopausal depression such as Hormonal treatment, antidepressant treatment, physical activities treatment, mindfulness-based therapies, cognitive behavioural therapies, and mind-body therapies.
| Symptoms of Postmenopausal Depression | |
|---|---|
| Early neurovegetative symptoms | Hot flushes, insomnia, night tachycardia (due to rapid dysregulation of the hypothalamic set points of oestrogenic fluctuations on neuronal functioning. |
| Early effective symptoms | Anxiety, depression (due to impact on the limbic system of both oestrogenic alterations which is the key to disrupting neurotransmitters such as serotonin, dopamine and endorphins as well as disruption by environmental stress across the menopause). |
| Late cognitive and motor symptoms | Expresses the long-term effect of oestrogen hormone loss and disrupt neuronal survival. |
5.3.1. The Hormonal (Oestrogen) Treatment
For postmenopausal depression may be efficacious in two conditions either to stabilize or restore the elevated homeostasis which basically occurrs in premenstrual, postpartum or perimenopausal conditions [151]. And the second condition is to act as a psychomodulator during the state of decreased oestrogen levels and an increase in the occurrence of dysphoric mood, as occured in postmenopausal women. There were many researches which suggest and provded evidence for that oestrogen may be used as ailment as compared with a sole antidepressant for depressed perimenopausal women [152, 153]. Most of the women are willing to use Hormone Replacement Therapy (HRT) during the menopause conditions as it may give higher benefits for mood and behaviour adjustment. As the treatment course of postmenopausal depression is insidious and more resistant to conventional antidepressants as compared with premenopausal women so it has shown better outcomes when antidepressants are used in combinations with the hormone therapy. It has been assumed that chronic elevation and hypo-oestrogenic conditions may reduce the response of antidepressant drugs such as SSRI (Selective Serotonin Reuptake Inhibitors- fluoxetine, citalopram, paroxetine). Based on DSM-IV criteria, 39 female patients in perimenopause and 22 in postmenopause who participated in prospective evaluation and are not on HRT, showed the effectiveness of antidepressant treatment for 6 weeks. After controlling for age, baseline symptom severity, antidepressant dosage and hormone levels of Follicle-Stimulating Hormone (FSH), luteinizing hormone (LH) and estradiol (E2), this treatment showed poor response in postmenopausal women as compared with the response of premenopausal women. Old age, mid-age and high levels of FSH are also associated with the efficacy of antidepressants in postmenopausal women. Old age and menopausal conditions are the predictors of poor response towards the antidepressant treatment. And furthermore, the FSH may interfere with the mechanism of action of antidepressant agents [154].
5.3.2. Physical Activity Treatment
Played a vital role in the ailment of postmenopausal depression because physical inactivity not only leads to women’s health risk but also increases menopausal problems. Many pieces of evidence are there which link habitual physical exercise (PE) and prevent and treat numerous ailments which typically occur in mid-life age onwards. And PE constitutes a form of therapy in itself [155] and lack of PE can lead to different types of disorders and problems.
The physical activities and exercise are especially useful when they are associated with other healthy lifestyle habits which help to increase their capacity and potential for reducing the stress and behaviour disorders and discomforts of menopause [156]. By the recommendations from the AHA (American Heart Association) and the American College of Sports Medicine (ACSM), PE are divided into four areas especially for older people are [157]:
5.3.2.1. Aerobic Physical Exercise
Walking, swimming, dancing, and cycling, basically involves the usage of large muscle groups and are sustained for at least 10 min but the AHA and ACMS suggest a minimum of 30 min of moderate aerobic activity 5 days/week or a minimum of 20 min of vigorous range about 3 days/week.
5.3.2.2. Muscle-Strengthening
Performed for 2 days/week and volunteers should be instructed to work for large groups of muscles such as abdomen, arms, legs, shoulders, and hips).
5.3.2.3. Flexibility
Is used to improve the tasks of everyday life and balance the problems and maintain stability and decrease risks [158].
The prescription of physical exercise given by the physician depends upon the individual comorbidities, general physical health and level of physical fitness. Most of the women with obesity and sarcopenia who experience instability during gait are encouraged to exercise which may lead to weight loss but increase in muscle mass. However, physical exercise increases their incidence of musculoskeletal injuries and destructions [159].
5.3.3. Mind-body Therapy
Is another therapy for the treatment of postmenopausal depression because it was estimated that 75-85% of women experience this disease and related symptoms [160], including vasomotor disturbances (hot flashes/night sweats), tiredness, sleep disturbances, cognitive disruption, musculoskeletal pain and headaches. Symptoms begin to arise at least 1 year before menstrual period cessation and persisted for several years postmenopause [161, 162]. Most of the women are turning to an increasing number of complementary and alternative therapies which help to manage menopausal symptoms. The commonly chosen therapies are yoga, meditation, mind-body therapies, tai chi, as well as stress relieving relaxation management techniques [163-165]. As menopausal symptoms contribute to exacerbation of psychosocial stress, so these therapies lift the mood, behaviour, stress reactivity reduction, improve sleep cycles and fatigue. These techniques are reported to decrease the induction of sympathetic activation, the vasomotor and other menopausal symptoms appeared and these factors play an important oetiologic role in the development of insulin resistance, dyslipidaemia, high blood pressure (hypertension), and other atherogenic changes which are associated with menopause [166-169]. Most of the open trials have shown effectiveness towards treating menopausal depression but there is a lack of clinical trials on them. Hormonal therapy (HT), including transdermal estradiol, has shown the effectiveness of the treatment of depression during the menopausal stage in women [170-172].
5.3.4. Cognitive and Mindfulness-based Therapies
Are the therapies which show better treatment efficacy in treating postmenopausal depression. Cognitive behavioural therapy (CBT), is an effective psychological and time-limited structured therapy given by physicians because these can show potential in research studies and are highly effective in reducing the symptoms related to mental health and related conditions [173]. Other psychological approaches are incorporated with the use of mindfulness meditation because they can increase the insight of human being, i.e. how automatic, habitual links and patterns of overidentification and cognitive reactivity towards sensations, emotions, feelings, and thoughts increasing stress and emotional distress. Mindfulness-based interventions are used to reduce the symptoms producing emotional well-being. Approaches such as Mindfulness-Based Stress Reduction (MBSR) and Mindfulness-Based Cognitive Therapy (MBCT) are successfully used for treating the depression, related to mental illness and physical conditions [174-177]. Cognitive behavioural therapy is useful in treating the perimenopausal disorder in women whose state of mind is influenced by difficult midlife stressors and negative thoughts and attitudes towards ageing. This therapy helps women to learn and counter their negative thoughts, solve the problems and actively participate in physical activities and also withstand the symptoms which lead them to feel isolated and unmotivated and lonely [178].
6. THE WINDOW OF VULNERABILITY AND PATHWAYS INVOLVED IN PPD AND POSTMENOPAUSAL DEPRESSION IN WOMEN
In pregnancy and after the menopause if the premenstrual syndrome is absent before puberty, it supports the theory of cyclical ovarian activity which is important in females. Various other events which include postpartum depression and the baby blues can show the relationship between depression and reproductive periods which can be described by Soares as a – ‘window of vulnerability’ [179].
6.1. Sleep Disturbances
Most of the females complain about difficulties initiating and or maintaining proper sleep during the menopausal transition. However, it is difficult to determine whether this is a primary disorder or it may be related to vasomotor symptoms of depression. It may also cause medical conditions and lifestyle. Sleep quality can be worsening if obstructive sleep apnoea and leg syndrome problems arise which is common in this age group [180]. The possible interactions between menopausal changes and sleep disturbances are investigated and are associated with the onset of sleep disturbances [181]. Vasomotor symptoms are the primary predictor of sleep problems in menopausal women while sleep disturbances were shown and associated with depression in midlife women when compared with non-depressed females, and those with VMS have even worse sleep quality and a high number of depressed groups [182, 183]. These were improved by using sleep aids such as zolpidem, with no improvement in flush number during the day time, suggesting that women were sleeping all night with no changes in nocturnal VMS.
6.2. Sexual Dimorphism
It was studied that sexually dimorphic behaviours may be suggestive of sex differences in brain neuroanatomy and neurophysiology [184]. The preoptic is the area which is responsible for reproduction, thermoregulation, and sleep with the help of a complex network of regulatory signals which include several neurotransmitters, neuropeptides and hormones.
Serotonin (5-HT) is one of the neurotransmitters which is involved in thermoregulation as well as sleep and release of Gonadotropin-Releasing Hormone (GnRH). Any type of changes occurred in 5-HT receptors and transporters are reported in ageing, and in animal studies in which preoptic neurons are affected during reproductive ageing [185]. The animal studies also demonstrated some behaviour associated with kisspeptin and its related receptors which include anxiety and depression. The alterations are shown by kisspeptin in the postmenopause period that may play a role in mood regulation in women during their midlife years [186].
6.3. Oestrogen Receptor Polymorphism
The oestrogen receptors are found throughout the brain, and the effects of oestrogen are observed in the hypothalamus, prefrontal cortex, and hippocampus and brainstem regions. As in animal models, oestrogens withdrawal has been proposed to play a key role in the onset of postpartum anxiety and depression which occurs mainly within 4 to 6 weeks after the birth of a child. However, circulating oestrogen levels are dramatically and drastically reduced during the early postpartum periods and then return to dioestrus levels at 3 weeks postpartum, raising the possibility that changes in oestrogen levels and its sensitivity are involved in postpartum depression [187].
Genetic association studies identified the individual candidate genes as well as potential pathways involved in postpartum depression.
6.4. HPA Pathways
Numerous findings implicate that the HPA axis is involved in postpartum depression. Variations in both the MAOA and COMT genes are implicated in postpartum depression and are also linked with sex-specific differences in cortisol responses to a social stressor. Protein Kinase C Beta Type (PRKCB) is a regulator of the HPA axis indirectly through Glucocorticoid Receptors (GR) and Corticotropin-Releasing Hormone (CRH) signalling and mutations in this gene are also associated with postpartum depression [188, 189].
6.5. HMNC1 (Hemicentin 1 Gene)
The Hemicentin 1 gene had the strongest association with postpartum depression. Though its function is unclear, HMNC1 is highly shown in the hippocampus region, which was shown to be altered in rats followed by a postpartum drop in oestrogen levels. Further studies were held which showed a relationship between HMNC1 and the postpartum depression, for which candidate gene approach was taken which confirmed that the HMCN1 polymorphism was associated with postpartum depression in a small number of Brazilian women. Further research required to find about the role of HMNC1 gene in underlying pathogenesis of postpartum depression [190-192].
7. CORRELATION OF OT AND HPA WITH PPD
7.1. Result and Discussion
Postpartum depression is a major international human health problem which affects at least one in eight mothers and their children in the year after parturition (childbirth) worldwide affecting the mother-infant relationship. It has been associated with negative effects and related symptoms not only on women who were depressed but also on their children affecting their physical, cognitive and emotional development and state of mind. Most and numerous studies show the effectiveness of the medication and various therapies.
A study was conducted showing the effect of oxytocin and HPA on PPD in women. Lactation has shown shielding action towards stress reactivity due to the release of oxytocin. In women suffering from postpartum depression, the Dysregulation of the HPA axis has been reported. On the other hand, the presence of PPD and failure to lactate has shown a strong correlation in the OT signaling thus leading to PPD. A research was performed on fifty pregnant women who anticipated breastfeeding. 47 out of them underwent Trier Social Stress Test (TSST) after eight weeks of postpartum whereas thirty nine women were breastfeeding at the time of conduction of TSST. The symptoms were noted and blood was withdrawn to check the hormone levels. It was found that in breastfeeding women the OT surge leads to shielding of the stress-induced secretions of CORT. A strong correlation was noted in symptomatic women who were breastfeeding and showed a correlation between OT, AUC and CORT. The results showed that in symptomatic breastfeeding women low OT levels were noted and high CORT levels were seen during breastfeeding during the TSST in comparison to the asymptomatic breastfeeding women [193].
Trials are held for medication specifically evaluating the effectiveness and potential capability of antidepressant medication, hormonal therapy or Electroconvulsive Therapy (ECT) for postpartum depression They also provided evidence that those treatments which show potential in treating major depression in the general population are equally effective in treating postpartum depression. Selective Serotonin Reuptake Inhibitor (SSRI) is mostly used in treating PPD in women. [194].
It is suggestive from all the cases reported that women of midlife age experience major episodes of depression especially during perimenopausal, postmenopause and postpartum phases. Depression of midlife is a classic case of depression commonly noted with symptoms like sleep disturbance, vasomotor symptoms etc. and even major psychological disorders. Symptoms of menopause co-occur thus making it a complicated case of depression [ 195 ].
Various diagnosis involves the prior detection of menopausal stage by assessment of the concurrently occurring symptoms of psychiatric and psychological conditions.
8. CURRENT FINDINGS
It has been estimated that around 20-30% of women who are suffering from depression (perimenopausal) are at a higher risk of CVD (cardiovascular diseases) and mortality. The therapeutic effects and withdrawals of estradiol (E2) have suggested that higher level sensitivity changes of E2 leads to perimenopausal depression [196] .
Second generation anti-psychotic drugs like dopaminergic agents, levothyroxine etc. were effective in the treatment of depression. Also it was found that administration of OTC drugs majorly nutraceuticals along with AD, provided a safer and effective therapy than the conventionally used ADs. Agents like saffron, S-Adenosyl-L-Methionine, N-acetylcysteine, NSAIDs, curcumin, ω-3 fatty acids, 5-hydroxytryptophan etc., caused augmentation of basic treatment of AD [ 197 ].
Irregular sleep cycles, e.g. circadian rhythm, were responsible for cognitive behaviour including mood swings, depressions etc. as disturbance of sleep is very common in pregnant women and even during postpartum. A study was performed on 51 women with previous non active, uni or bipolar depression symptoms, and circadian phases were recorded. It was noted that timing and duration of sleep is a modifiable factor which can help in the treatment of depression in women as the late sleep group was reported to be with maniac, OCD as well as depressive behaviours [ 198 ].
A GABAergic neurosteroid Allopregnanolone is a derivative of progesterone which has been lately approved by Food and Drug Administration for treating postpartum depression. Neuroendocrine dysfunction, neurotransmitter alterations, neuroinflammation, epigenetic and genetic modifications etc. are involved in the pathogenesis of PPD. In recent studies, it was revealed that mothers consuming unhealthy diets are probably at a higher risk of PPD. As the microbiome composition is responsible for increasing inflammation, that’s why it is strongly related to mood disorders. Thus functional foods like omega-3 fatty acids, vitamins, minerals and flavonoids play a very important role in preventing neuroinflammation hence controlling depression. The functional foods improving inflammation conditions can be linked with peroxisome-proliferator activated receptor (PPAR) pathways thus regulating the biosynthesis of allopregnanolone and neurotropic factors (Brain derived neurotropic factors- BNFD) thereby improving mood. Therefore, it was concluded that functional foods lead to regulation of neurosteroids [199, 200].
CONCLUSION
As the hormonal balance is deeply fluctuating in women during their critical times like menopause, premenopausal, pregnancy or post-delivery etc. hence the symptoms of depression are visible in such transition periods. Accumulating these conditions can lead to clinical depression. As the major reason behind this depression is hormonal imbalance. Hence as per the research conducted, the best suited therapy for treating critical periods like postmenopausal, premenopausal and postpartum depression, is hormonal therapy (oestrogen and transdermal estradiol). Women must be careful of anti-depressant medication during their pregnancy and breastfeeding phases. More detailed studies are required to understand and overcome other related factors which could possibly elevate the symptoms. As a strong concern over women health issues is arising, it will be a relief to spread awareness on women health and also obtain medical therapy for treatments.
LIST OF ABBREVIATIONS
| MCR | = Metabolic Clearance Rate |
| DHEA | = Dihydroepiandosterone |
| CBG | = Cortisol Binding Globulin |
| CRH | = Corticotropin-releasing Hormone |
| ACTH | = Adrenocorticotropin (Corticotropin) Hormone |
| T3 | = Triiodothyronine |
| T4 | = Tetraiodothyronine (thyroxine) |
| TSH | = Thyroid Stimulating Hormone |
| TBG | = Thyroid Binding Globulin |
| LH | = Luteinizing Hormones |
| FSH | = Follicles Stimulating Hormone |
| hCG | = Human chorionic gonadotropin (placental hormone). |
| HPA | = Hypothalamic Pituitary Adrenal |
| TSST | = Trier Social Stress Test |
| OT | = Oxytocin |
| CORT | = Cortisol |
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors are thankful to the Pharmacy Department of Pranveer Singh Institute of Technology, Kanpur, Uttar Pradesh, India, who helped us a lot while writing this review article.