All published articles of this journal are available on ScienceDirect.
Human Amniotic Fluid for the Treatment of Hospitalized, Symptomatic, and Laboratory-verified SARS-CoV-2 Patients
Abstract
New reports offer evidence that under different circumstances, intrauterine mother-infant transmission of SARS-CoV-2 occurs. In contrast, early observations in the COVID-19 pandemic recommended that vertical transmission from women infected with SARS-CoV-2 can be challenging and no virus is detected in human amniotic fluid (HAF). The present study aimed to propose the idea that HAF can be used as a potential therapy for hospitalized, symptomatic, and laboratory-verified SARS-CoV-2 patients by mitigating COVID-19 related inflammation and decreasing its fibrosis.Considering that COVID-19 can cause a severe pulmonary fibrotic response in some patients, HAF by decreasing fibrosis may be considered as an alternative and novel therapy against COVID-19. Lastly, given the inexpensive, easy to access, and safe nature of HAF, integrating this therapy may decrease the COVID-19 attributed death and burden to the health system, especially in countries with limited access to vaccines where HAF is widely available.
1. INTRODUCTION
SARS-CoV-2, the virus that causes COVID-19, has emerged recently and spread rapidly to 219 countries and territories, causing over 103,985,844 infections with 2,249,445 deaths so far [1]. The fatality rate of COVID-19 disease was initially reported to be 15%, but this estimate was calculated from a small group of hospitalized patients. After that, with the emergence of more data, the fatality rate of this disease was reported between 3.4% to 11% and later reached 3 to 3.4% [2]. A distinctive feature of the SARS-CoV-2 is its high contagiousness and rapid spread between people, which has led to a severe outbreak of the COVID-19 disease [3]. On the other hand, the 2 to the 14-day incubation period of the SARS-CoV-2, high transmission ability, and similarity of its symptoms with the common cold have led to the greater COVID-19 prevalence in the world [4]. Patients with infection with positive clinical signs that are commonly suspected of having the COVID-19 disease are often presented to medical centers with symptoms, such as fever, dry cough, shortness of breath, and diarrhea [5]. New reports offer evidence that under different circumstances, intrauterine mother-infant transmission of SARS-CoV-2 occurs [6, 7]. In contrast, early observations in the COVID-19 pandemic recommended that vertical transmission from women infected with SARS-CoV-2 can be challenging and no virus is detected in human amniotic fluid (HAF) [8, 9]. Therefore, it seems necessary to introduce novel treatments for this virus as a global problem. These pieces of evidence raise questions about whether there is something in HAF that keeps patients from being harmed. HAF products have an extensive immunity and safety profile modulated by physiologic and pathologic processes [10]. The best way to fight the COVID-19 pandemic is vaccination, which may significantly reduce the risk of developing the disease and its serious consequences by creating an immune response. Because COVID-19 is an unknown and new disease, the study of effective treatments and drugs for this disease is one of the major health challenges [11]. Studies on treatments for this disease are still in the early stages, but there is ample evidence that several treatments and medications have been effective [12]. Furthermore, the justification for evaluating a new treatment because of the unavailability of vaccines seems inadequate. Early treatment is a form of secondary prevention, while vaccination is a form of primary prevention, therefore, treatment does not replace the need for a vaccine, as they are measures for completely different clinical situations. This may shed light on the need for novel and effective treatment apart from the availability of vaccines against COVID-19 disease.
The present study aimed to propose the idea that HAF can be used as a potential therapy for hospitalized, symptomatic, and laboratory-verified SARS-CoV-2 patients by mitigating COVID-19 related inflammation and decreasing its fibrosis.
2. COVID-19 DIAGNOSTIC METHODS BASED ON SEROLOGICAL AND MOLECULAR TECHNIQUES
Both serological and molecular techniques are the most widely used diagnostic methods for COVID-19 infection compared to other methods [13]. The number of different blood cells, including leukocytes, lymphocytes, neutrophils, platelets, and hemoglobin, undergo changes that can indicate the type and severity of the COVID-19 disease [14]. For COVID-19 serological analysis, ELISA kits containing viral nucleoproteins can also be used to detect immunoglobulins, such as IgM and IgG [15]. Apart from ELISA-based methods and kits, turbidimetric, nephelometric, immuno-electrophoresis and other serological methods use to measure IgM and IgG produced in COVID-19 disease [16]. The molecular study of COVID-19 based on the RT-qPCR technique is an enzymatic method for the production of a large number of target genes [17]. Despite the importance of accelerating the process of identification and control of COVID-19 disease and the need to use rapid diagnostic tests, it is also necessary to follow rapid diagnostic tests simultaneously with molecular techniques to identify patients with COVID-19 infections [18].
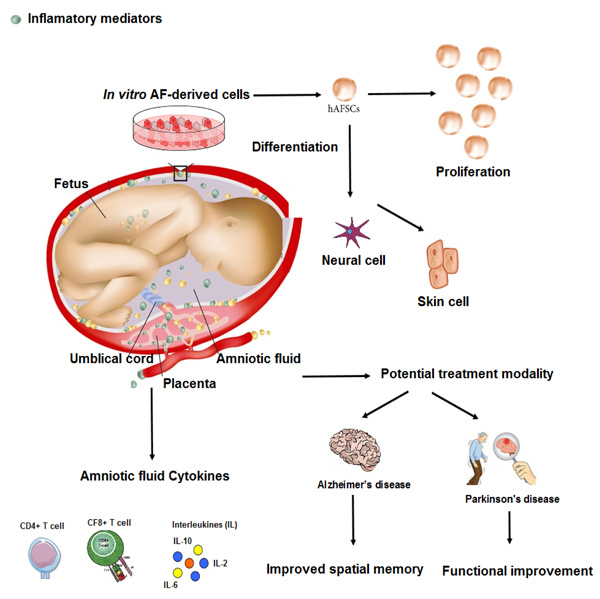
3. BIOLOGICAL AND CLINICAL PLAUSIBILITY OF USING AMNIOTIC FLUID
Recent reports showed that HAF has anti-inflammatory [19], antibacterial [20], and regenerative activities [21], possibly through gene expression of factors involved in osteoarthritis and antibacterial proteins, such as cystatin C and lactoferrin. In a new study, researchers conducted experiments to gain further insights into the therapeutic potential of amniotic fluid-derived stem cells and showed that this amniotic fluid derivative was able to activate cell survival and apoptosis pathways. Amniotic epithelial cells not only express surface antigens and markers of embryonic stem cells, such as SSEA3, SSEA4, TRA1-60, and TRA1-80 [22] but also express stem cell-related factors, such as octamer-binding protein 4 (OCT4) and Nanog [23]. These cells also show the ability to differentiate into three germ layers, including mesoderm, ectoderm, and endoderm [24]. Amniotic fluid-derived stem cells are a good source of cell regeneration due to their high differentiation potential and anti-inflammatory properties [25]. In addition, these cells have immune-modulatory properties that inhibit the immune response and are not tumorigenic [26]. Researchers are now looking at a promising approach using amniotic fluid-derived stem cells to treat various diseases, especially chronic diseases. Using the unique properties of amniotic fluid-derived stem cells, these researchers have tried to recover the function of body organs in a pre-clinical model (Fig. 1). Their results show that this type of cell can be a general source of stem cells and provide an alternative treatment strategy for various diseases [27]. In another study [28], researchers conducted experiments to gain further insights into the therapeutic potential of amniotic fluid-derived stem cells and the pathways that are activated by the secretome of these stem cells. They examined the exosomal fraction present in the environment on these cells and the microRNA content within these exosomes. The secretory derived from HAF-stem cells was able to activate cell survival and anti-apoptosis pathways. MicroRNA analysis in exosomes revealed a panel of more than 16 microRNAs involved in survival signaling pathways, including neurotrophin signaling, which is strongly associated with neuroprotection and neuronal cell death. The results show that exosomes derived from HAF have neuroprotective effects and can increase the survival of nerve cells.
4. HOW WOULD THE PROTECTIVE FACTORS DESCRIBED IN THE AMNIOTIC FLUID ACT IN THE PATHOPHYSIOLOGY OF COVID-19?
The knowledge of the pathophysiology and treatment of COVID-19 is growing rapidly. Patients with COVID-19 show a wide range of symptoms, from the disease as a cytokine-storm syndrome with a highly aggressive inflammatory response to patients with a less inflammatory response [29]. Limited information is available on the use of HAF for cardiopulmonary disease. Many COVID-19 patients, in addition to developing acute respiratory distress syndrome (ARDS), lung fibrosis, and severe hypoxia [30], serious cardiovascular manifestations, including myocarditis, heart failure, and arrhythmia [31]. Therefore, HAF can affect the natural history of the COVID-19 disease by reducing inflammation and pulmonary fibrosis. Recent research has also opened new windows on using HAF to repair blood vessels in cardiovascular disease (CVD) [32], which can also be extended in the context of COVID-19 treatment.
5. LIMITATION
Due to the lack of knowledge on the details of cellular stages or viral replication stages in association with the use of human amniotic fluid, we cannot say that at what stage of viral replication or contamination of human cells the effect of human amniotic fluid would occur.
CONCLUSION
This study shed light on the need for a larger-scale randomized prospective trial to advance examine HAF as a safe therapy for hospitalized, symptomatic, and laboratory-verified SARS-CoV-2 patients. Generally, HAF is a safe and diverse mixture of elements, of which a large-scale gene expression of 64,000 genes associated with HAF was demonstrated in term gestation patients [10, 33]. Moreover, a study on cytokine array from term gestation patients showed that 300 proteins out of 400 with host defense and angiogenesis effects were present in HAF [33]. Outside of the mentioned researches, there is reported data concerning the use of HAF-derived stem cells on lung fibrosis and regeneration [34-36]. Considering that COVID-19 can cause a severe pulmonary fibrotic response in some patients [37], HAF by decreasing fibrosis may be considered as an alternative and novel therapy against COVID-19. Lastly, given the inexpensive, easy to access and safe nature of HAF, integrating this therapy may decrease COVID-19 attributed deaths and burden to the health system.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors are grateful to Assistant Prof. Dr. Najmieh, who has been extremely helpful.


