Different Effects of Fluoxetine and Paroxetine Combined with Vitamin D3 in Ovariectomized Rats Exposed to Unpredictable Stress
Abstract
Background:
Vitamin D3 (VD3) is involved in the pathophysiological mechanisms of affective-related disorders and controls the functional activity of various hormonal systems. The complex interaction between estrogen and VD3 creates a neurobiological basis for their participation in similar behavioral disorders.
Objectives:
This study aimed to evaluate whether VD3 (5.0 mg/kg, s.c.) facilitates the antidepressant-like action of fluoxetine (10.0 mg/kg, i.p.) or paroxetine (10.0 mg/kg, i.p.) by enhancing the antidepressant-like activity of these drugs in adult long-term Ovariectomized (OVX) rats subjected to Chronic Unpredictable Mild Stress (CUMS) protocol for 6 weeks.
Methods:
Sucrose Preference (SPT) and Forced Swim (FST) tests were performed to evaluate the anhedonia state and depressive symptoms, respectively. The Open-Field Test (OFT) was carried out to measure locomotor activity as well as grooming behavior produced by CUMS in long-term OVX rats. Corticosterone (CS)/estradiol (E2) in the serum was tested by rat ELISA kits. NF-kB, 5-HT/5-HIIA, and pro-inflammatory cytokine levels in the hippocampus were also examined by rat ELISA kits.
Results:
The results of this study suggest that combined treatment with fluoxetine (10.0 mg/kg, i.p.) or paroxetine (10.0 mg/kg, i.p.) along with VD3 (5.0 mg/kg, s.c.) produces distinct effects on the depression-like behavior in long-term OVX/CUMS rats. Co-administration of fluoxetine (10.0 mg/kg, i.p.) with VD3 did not facilitate the antidepressant-like effects of fluoxetine in the long-term OVX rats with CUMS. On the other hand, co-treatment with paroxetine with VD3 resulted in faster and marked antianhedonic- and antidepressant-like effects in long-term OVX rats with CUMS as compared to treatment with paroxetine alone. The co-administration of paroxetine and VD3 attenuates stress-induced modifications of CS/E2 levels in the serum, as well as- proinflammatory cytokine/NF-kB/5-HT levels in the hippocampus of long-term OVX rats exposed to CUMS.
Conclusion:
Supplementation of VD3 (5.0 mg/kg, s.c.)to paroxetine (10.0 mg/kg, i.p.) facilitates antianhedonic- and antidepressant-like effects of paroxetine in adult long-term OVX rats exposed to CUMS.
1. INTRODUCTION
Estrogen deficiency during menopause can lead to profound endocrine disorders and neuropsychiatric symptoms, such as memory impairment, insomnia, anxiety, and depression [1-3]. These disorders have an adverse impact on the quality of life [4]. Approximately 36% of the menopausal women report experiencing depressive symptoms [5].
Despite numerous neuropharmacological schedules and psychological rehabilitation approaches to treat mood disorders, only moderate improvements have been registered in the treatment of affective-related disorders. More importantly, the possibility of recurrence is increased especially in women during estrogen deficiency [6, 7]. The standard treatment of mood disorders with antidepressants highlights the restricted effectiveness of the current approach [8-11].
Among the different classes of antidepressants, SSRIs are the most extensively used pharmacological drugs to treat affective-related disorders in perimenopausal and postmenopausal women [3, 9, 11]. However, the currently used antidepressants are not completely effective and have many adverse effects [6-8]. Moreover, the therapeutic response to SSRIs in perimenopausal women might be influenced by modifications in the concentrations of female gonadal hormones that can restrict their application in such cases [12, 13]. The absence or delayed response to treatment with antidepressants is a major problem in the therapy of depression [8-11].
The monoamines hypothesis of depression suggests that depression arises from the decreasing release of monoamines, especially serotonin (5-HT) in the brain [14]. Another important factor behind the onset of depression is inflammation in the brain [15-19]. Depressed patients have been documented to have higher levels of serum pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-6, and tumor necrosis factor-alpha (TNF-α) [19, 20]. Moreover, increased inflammatory mediator levels may act as predictive biomarkers to resist the pharmacotherapy of depression [21]. Furthermore, nuclear factor-kappa B (NF-κB) is one of the most important pro-inflammatory transcription factors that is involved in the pathophysiology of numerous peripheral and central inflammatory impairments [22, 23]. NF-κB is activated by stress and might mediate molecular outcomes to stress factors and trigger mood deteriorations [24, 25]. Findings from clinical studies indicated that women in the menopausal period have a significant imbalance in the production of inflammatory molecules as well as decreased serotoninergic activity [26, 27].
Vitamin D (VD) deficiency is one of the many factors that lead to the onset of mood disturbances [28]. VD is synthesized in the skin and can be also found in food [29, 30]. A strong relationship between VD deficiency and profound affective-related state, as well as between VD deficiency and cognitive function have been reported in several studies [31-34].
Estrogen imbalance can lead to symptoms of VD deficiency since estrogen induces the increased activity of the enzyme system causing VD action [35]. Some clinical studies showed that mild depressive symptoms are more frequently registered in women with VD insufficiency, and even more so in women with VD deficiency [36, 37]. In addition, VD3 controls the functional activity of the 5-HT system in the brain [38]. VD3 might transcriptionally activate the tryptophan hydroxylase-2 gene, resulting in increased conversion of tryptophan to 5-HT in the brain [38, 39]. Moreover, VD3 modulates neuroinflammation in the brain and decreases proinflammatory cytokine levels in the central and peripheral nervous system [40, 41].
Altogether, the above-mentioned findings suggest that mood disturbances in menopausal women may involve complex deterioration in estrogen and VD3 levels, neuroinflammation, as well as abnormal 5-HT levels in the brain.
In our previous studies, we assessed the antidepressant-like effects of VD3 at different doses (1.0, 2.5, and 5.0 mg/kg, s.c.) on a model of depression induced by Chronic Unpredictable Mild Stress (CUMS) for 28 days in adult ovariectomized (OVX) rats [42]. The results of our study demonstrated that VD3 (5.0 mg/kg, s.c.) considerably reversed the depression-like state in the Forced Swimming Test (FST) in long-term OVX rats compared to OVX rats with CUMS [42]. Therefore, we designed the current study taking into account the antidepressant-like activity of VD3 in the affective-related profile of the long-term OVX females.
This study aimed to evaluate whether VD3 facilitates the antidepressant-like action of fluoxetine or paroxetine by enhancing the antidepressant-like activity of these SSRIs in female rats with long-term deficiency of estrogen. Fluoxetine and paroxetine are well-known SSRIs that are frequently prescribed in the treatment of depression [43, 44]. Moreover, paroxetine is also suspected to modulate the female reproductive system and estradiol levels in postmenopausal women [45, 46]. The effects of co-administration of fluoxetine or paroxetine with VD3 were evaluated by the sucrose preference test, forced swimming test, and open-field test in adult female rats with long-term deficiency of estrogen exposed to the CUMS. The effects of co-administration with VD3 and fluoxetine/paroxetine on changes in NF-kB signaling, pro-inflammatory cytokines, and 5-HT levels in the hippocampus were evaluated in the adult female rats with long-term deficiency of estrogen exposed to the CUMS.
2. MATERIALS AND METHODS
2.1. Animals
Female Wistar rats (190 ± 20 g) were purchased from the Animal Rat Center at the Rappolovo Laboratory Animal Factory (St. Petersburg, Russia). A total number of 56 female rats at the age of 3 months at the beginning of the experiment were divided into different groups. Rats were housed singly at a controlled temperature (22 ± 1 °C), humidity (50 ± 10%), and light conditions with adlibitum access to food and water. The light was programmed on a 12h light/dark cycle (lights on at 7:00 AM). The experimental females were assigned to stay at the animal room for 1 week before their use in the experiment. All procedures with animals were carried out in compliance with national and international laws and policies. Experimental rats were decapitated with anesthesia for brain sampling and every effort was made to minimize the pain experienced by the animals. In all stress procedures, the researchers treated the rats with care and were sensitive to their emotional state.
2.2. Surgery Protocol
Ovariectomy was performed as previously described [42]. The rats were anesthetized with narcosis that comprised of a mixture of 10 mg/kg xylazine and 70 mg/kg ketamine for bilateral removal of the ovaries. Ovariectomy was performed using 2 typical cuts in a lateral position. Under anesthesia, a longitudinal incision was made along the dorsal midline. The muscle layer was drawn from the abdomen through the incision with sterilized hemostatic forceps. The oviducts were clamped with hemostatic forceps and the ovaries were removed. The muscle layer was then closed. The efficiency of the surgery was examined by routine vaginal inspection. The sham ovariectomy was performed in an identical way but without the removal of the female endocrine glands. After the ovariectomy and sham operation, the experimental rats were maintained in their home cage and were allowed to recover for a period of 3 months as a post-ovariectomy period. During the post-surgery interval, experimental groups of females admitted were given free access to food and water. After 3 months of the post-ovariectomy period, rats were assigned randomly to the experiment for chronic stress procedure, except for the control sham-operated (SHAM) rats.
2.3. Chronic Unpredictable Mild Stress Trial
The Chronic Unpredictable Mild Stress (CUMS) protocol was performed as reported previously [47, 48]. Rats in CUMS groups were submitted to different stressful stimuli for 14 days, followed by 28 days (from third to the sixth week) of VD3, fluoxetine, paroxetine, VD3, and fluoxetine, and VD3 and paroxetine treatment for which the stress protocol continued. The following stressors were used: water deprivation (24 h), food deprivation (24 h), wet bedding (24 h), tilted cage at 45° (24 h), a reversal of the light/dark cycle (24 h), swimming in cold water (4 °C, 5 min), swimming in warm water (45 °C, 5 min), and clippedtail (1 min, 1 cm from the end of the tail). The rats were exposed to one of the stressors on a given day. All stress factors were applied individually and continuously throughout the day. To avoid habituation, the same stressor was not applied consecutively for two days to guarantee that the animal does not predict the occurrence of any stress trigger. CUMS protocol was similar every time to maintain reproducibility. During the stress trial, the rats were placed into the experimental room to undergo the stress procedure and were returned to their single cages afterwards. The stress procedure did not involve any food or water deprivation in the present study. The females after sham-operation (control rats) were placed in a separate cage and vivarium section without any contact with the CUMS groups of animals for 6 weeks. The rats were generally undisturbed and well maintained (provided with food and routine cage cleaning). All experimental females were supervised daily by the veterinary care staff during subroutine maintenance. The rats were examined in a special animal room using a non-invasive observational assessment procedure that yielded information regarding the health of each animal. The assessment consisted of several measurements, such as body condition, appearance, breathing, hydration status, posture, mobility, muscle tone, as well as any presence of defects in the bones, genitals, and abdomen. No rats were injured or unhealthy during the experimental protocol.
2.4. Drug Treatments
The pharmacological preparations used in the current study were fluoxetine hydrochloride, paroxetine hydrochloride, and cholecalciferol as VD3. All of the drugs were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Fluoxetine hydrochloride and paroxetine hydrochloride were given Intraperitoneally (IP). VD3 was administered Subcutaneously (SC) in a volume of 0.1 mL/rat, respectively, for 21 days during the CUMS procedure: 1 hour before the daily stressor action, and again during the period of the behavioral testing. Behavioral evaluations were recorded 60 minutes after the final drug treatment. Fluoxetine hydrochloride and paroxetine hydrochloride were dissolved in sterile 0.9% saline daily. Cholecalciferol was dissolved in a solvent of 95% ethanol, and then aliquoted and maintained at –80°C. Cholecalciferol solution for SC injection was freshly prepared. VD3 was diluted in physiological saline water, resulting in a solvent of VD3 with 1% ethanol.
2.5. Groups of Animals
All animals were randomly assigned to a total of 8 experimental groups (n = 7 in each):
(1) sham-operated (SHAM) rats without the CUMS model treated with solvent (control).
(2) SHAM rats exposed to CUMS treated with solvent.
(3) long-term OVX rats exposed to CUMS treated with solvent.
(4) long-term OVX rats exposed to CUMS treated with VD3 (5.0 mg/kg/day, SC).
(5) long-term OVX rats exposed to CUMS treated with fluoxetine (10.0 mg/kg/day, IP).
(6) long-term OVX rats exposed to CUMS treated with fluoxetine and VD3.
(7) long-term OVX rats exposed to CUMS treated with paroxetine (10.0 mg/kg/day, i.p.).
(8) long-term OVX rats exposed to CUMS treated with paroxetine and VD3.
In preliminary our works, no significant differences were found between SHAM/OVX rats treated with physiological saline (0.9% saline) as the solvent for fluoxetine, and SHAM/OVX females treated with a special solvent for VD3 in behavioral trials (data are not shown). Therefore, physiological saline was used as the solvent for SHAM/OVX female rats. The doses of VD3 were based on our previous studies on the behavioral effects of VD3 on depression-like behavior of non-stressed long-term OVX female rats [42]. Doses of fluoxetine and paroxetine were chosen according to reported experimental data [49, 50]. All behavioral measurements were recorded 60 minutes after the last drug administration. To minimize animal suffering, all groups of rats were euthanized by pentobarbital overdose after all behavioral trials. The timeline of this experimental study is presented in Fig. (1).
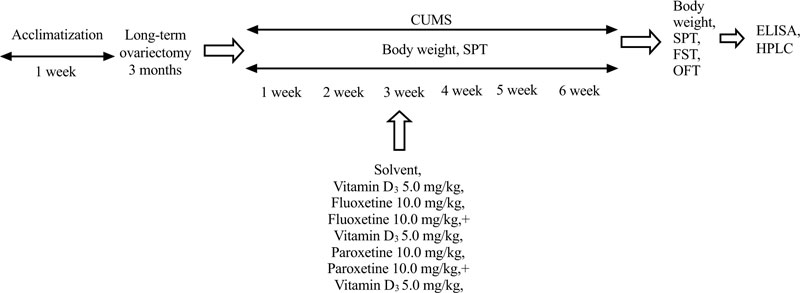
2.6. Body Weight
The body weight of all experimental groups was evaluated every week during the whole CUMS protocol.
2.7. Sucrose Preference Test
Sucrose Preference Test (SPT) was conducted every week during the 6 weeks of the CUMS protocol [44]. The SPT was performed as reported previously [42]. All groups of experimental rats were exposed to a 24-hour deprivation of food and water. After twenty-four hours, the subjects had 1-hour access to a bottle of water and a bottle of sucrose solution of 200 ml each. The measurement of sucrose preference was calculated using the following formula: % sucrose preference = sucrose intake/sucrose intake plus water intake × 100%.
2.8. Forced Swimming Test
The Forced Swimming Test (FST) was conducted as described previously [42]. During the FST test, the experimental female rats were put into the FST cylinders (height 60 cm and diameter 20 cm) that were filled with 23-25 °C water up till a depth of 30 cm. The immobility time, swimming time, and climbing time were recorded for 5 minutes using a video camera that was placed above the apparatus.
2.9. Open Field Test
The Open Field Test (OFT) was carried out as previously performed [42]. In brief, the animals were set in the center square of the OFT apparatus and observed for 300 seconds. The number of total crossings, the number of crossings in the central squares, the number of rearings, the time spent in the central squares, and grooming were measured for 300 seconds in the OFT using a video camera. After each animal’s trial, the OFT apparatus was cleaned thoroughly with 75% alcohol to prevent smell interference between trials.
2.10. Serum Corticosterone and Estradiol Measurements
Following behavioral tests, all rats were decapitated under deep anesthesia (10 mg/kg xylazine and 70 mg/kg ketamine) for brain sampling. 5 mL samples of blood were collected from the animals and were centrifuged at 4000 ×g for 15 minutes at 4°C. The brain tissue samples needed for ELISA assay were stored in a -80°C refrigerator for later use. The serum samples were used to estimate the Corticosterone (CS) and Estradiol (E2) levels using commercially available rat ELISA kits (Cusabio Biotech Co., Ltd, Wuhan, China) according to the manufacturer’s directions. The sensitivity and detection range of the CS were 0.1 ng/mL and 0.2-40 ng/mL, respectively, and 40.0 pg/mL and 40.0-1500 pg/mL for E2, respectively.
2.11. Hippocampal NF-kB/p50/p65, TNF-a, IL-1β, IL-6 Levels Determination
The cold lysis extraction buffer (0.2% sodium deoxycholate, 0.5% Triton X-100, 1% NP-40, 50 mM Tris-HCl pH 7.4, 1 mM phenylmethylsulfonyl fluoride, 1 mM N-ethyl-maleimide, and 2.5 mM phenanthroline) was used for the dissection and homogenization of the hippocampi of the experimental groups of rats [51]. The brain samples with cold lysis buffer were then sonicated for 15 seconds. After that, the hippocampi were centrifuged at 12,000 ×g for 15 minutes at 4°C. To normalize the hippocampi supernatants to the total protein, the Bradford method was applied [52]. The protein normalized supernatants of the hippocampus were stored at −80°C until assays were conducted.
Homogenates of the hippocampus in all experimental groups were utilized for evaluation of the levels using rat nuclear factor-kB/p50/p65 (NF-kB/p65/p50), tumor necrosis factor-alpha (TNF-a), interleukin-1-beta (IL-1β), interleukin-6 (IL-6) ELISA kits (Cusabio Biotech Co., Ltd, Wuhan, China) in accordance with the manufacturer’s recommendations. The NF-kB/p50/p65, TNF-a, IL-1β, IL-6 levels were detected using an MC Thermo Fisher Scientific reader (Thermo Fisher Scientific Inc., Helsinki, Finland) at 450 nm. The NF-kB/p65/p50 content is presented as pg/mg of tissue. The sensitivity and detection range of the NF-kB/p65 rat ELISA kit were 10.0 pg/ml and 62.5 pg/ml-2000 pg/ml, respectively. The sensitivity and detection range of the NF-kB/p50 rat ELISA kit were 0.119 ng/ml and 0.312 ng/ml–20 ng/ml. The TNF-a, IL-1β, IL-6 levels were expressed as pg/mL. The sensitivity and detection range of the TNF-a rat ELISA kit were 1.56 pg/mL and 6,25-400 pg/mL, respectively; for IL-1β rat ELISA kit - 1.56 pg/mL and 6.25-;4000 pg/mL, respectively; for IL-6 rat ELISA kit - 0.078 pg/mL and 0.312-20 pg/mL, respectively. The determination showed no significant cross-reference with other biological factors. The duplication of all samples was performed for the ELISA assay.
2.12. Hippocampal 5-HT and 5-HIIA Levels Measurement
The 5-HT/5-HIAA levels in the hippocampus were examined using High-Performance Liquid Chromatography (HPLC) as previously reported [53]. All hippocampal samples were homogenized in a 0.1 mol/L solution of HClO4 along with 0.02% Na2S2O2 (15 µL of solution for each mg of tissue) and Dihydroxybenzylamine (DHBA, 146.5 ng/mL, internal standard). These homogenates were centrifuged for 40 minutes at 11,000 ×g at 4°C. The isocratic system (Shimadzu LC-10AD, Kyoto, Japan) in HPLC equipment was supplied by a Spheri-5 RP-18 5 µm column (220 × 4.6 mm) and a 20 µL injection loop. The electrochemical detection was 0.75 V. The mobile phase consisted of 0.06% heptane sulphonic acid and phosphate/citrate pH 2.64, 0.02 mol/L, 0.12 mmol/L ethylene diamine tetraacetic acid, containing 10% methanol. The flow rate was 1 mL/min. 5-HT and its metabolite levels were expressed as mean ± SD ng/mg tissue. A solution containing 2 mL of sodium tetraborate 0.1 mol/L and 1 mL of stock solution was prepared before the assay of hippocampi. To finalize the precolumn derivatization, we used 100 µL of this solution with 50 µL of the sample for 2 minutes before each injection [53]. For the mobile phase, sodium phosphate 0.05 mol/L (pH 5.95) with 11.5% methanol were applied. HPLC system had a flow rate of 3.5 mL/min. The emission was detected at 460 nm, and excitation was detected at 348 nm. Standards of 5-HT and 5-HIIA were validated before determination. We did not find any additional peaks except 5-HT or 5-HIIA. The retention time was simultaneously detected for -HT and 5-HIIA.
2.13. Statistical Analysis
All experimental values are presented as mean ± Standard Error of the Mean (SEM). The differences in body weight and sucrose preference consumption among all experimental groups were analyzed using the two-way analysis of variance (two-way ANOVA) test with repeated measurements between subject factors for the duration and drug treatments. The other behavioral and biochemical evaluations between all experimental groups were analyzed using one-way ANOVA without repeated measurements followed by the post hoc Tukey test for multiple comparisons. The statistical analysis was carried out using the Statistics Package for SPSS, version 18.0 (SPSS Inc., USA). The probability level of P < 0.05 was considered to be statistically significant.
3. RESULTS
3.1. Combined Treatment (SSRIs with VD3) Increases Body Weight in Long-term OVX Rats Exposed to CUMS
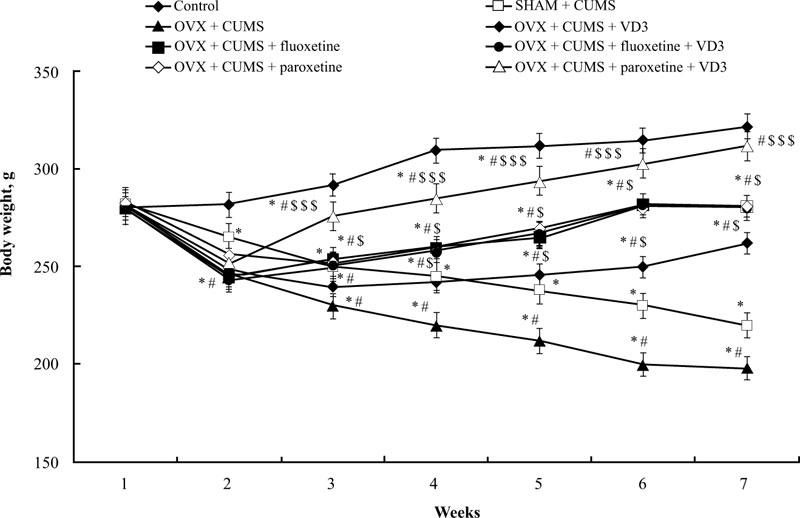
Before the CUMS protocol, we noted no significant difference in bodyweight among all experimental groups (Fig. 2). After 14 days of the CUMS trials, the ANOVA test indicated a significant effect of treatment (F(5,34) = 15.44, p < 0.05), effect of duration (F(5,34) = 11.09, p < 0.05), and a statistical effect of duration × treatment interaction (F(5,34) = 9.08, p < 0.05) on the body weight of the experimental groups. The difference in body weight among the groups (p < 0.05) was found using the post-hoc test. The body weight of SHAM rats subjected to CUMS gradually decreased after 2 weeks of paradigm protocol compared to non-CUMS group (post-hoc test, P < 0.05, (Fig. 2)). After 6 weeks of the CUMS procedure, the body weight of OVX/CUMS rats significantly reduced compared to the control non-CUMS and SHAM/CUMS groups (P < 0.05, (Fig. 2)). Following 28 days of different treatments, the body weight of OVX/CUMS rats treated with VD3 (5.0 mg/kg, s.c.), fluoxetine (10.0 mg/kg, i.p.) or paroxetine (10.0 mg/kg, i.p.) was significantly higher compared to OVX/ CUMS/solvent and SHAM/CUMS/solvent rats (P < 0.05, (Fig. 2)). The combination of fluoxetine along with VD3 (5.0 mg/kg, s.c.) failed to influence the body weight gain of OVX/CUMS rats as compared to the OVX/CUMS/fluoxetine rats. However, the two-way ANOVA revealed a significant increase in body weight in long-term OVX rats treated with paroxetine and VD3 after 1 week of therapy (at the 4th week of CUMS protocol) and maintained the same results in the following weeks as compared to the OVX/ CUMS/ solvent /paroxetine and SHAM/CUMS/solvent rats (P < 0.05, (Fig. 2)).
3.2. Combined Treatment (SSRIs with VD3) Restores Sucrose Preference in Long-term OVX Rats Exposed to CUMS
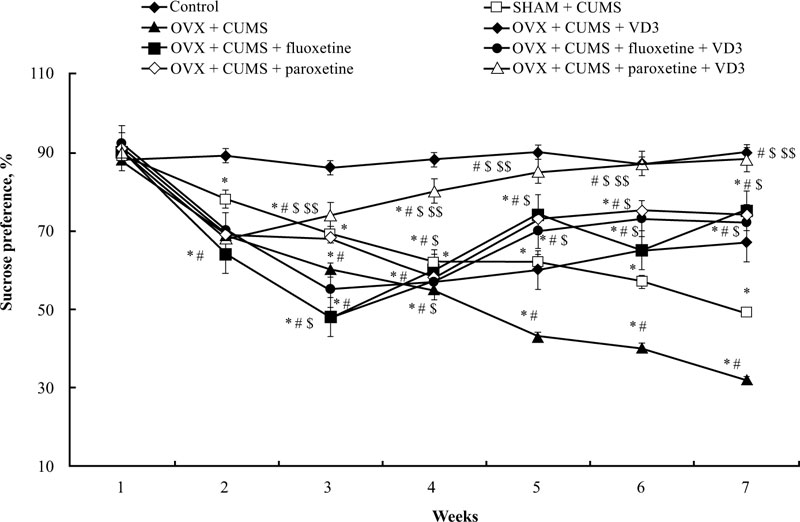
The sucrose preference of long-term OVX rats subjected to CUMS and treated with SSRIs in a combination with VD3 is presented in Fig. (3). A two-way ANOVA revealed significant differences in the sucrose preference between duration (F(5,34) = 11.41, P < 0.05), between drug treatment (F(5,34) = 15.07, P < 0.05), and an interaction between duration and treatments (F(5,34) = 2.98, P < 0.05) in the long-term OVX/CUMS rats. The post-hoc test revealed differences among the groups for sucrose preference (P < 0.05). We found no difference in the initial sucrose preference in all the experimental groups (P > 0.05, (Fig. 3)). Following CUMS trials, all experimental rats exhibited a decrease in sucrose preference in the 3rd week when compared with the control non-CUMS SHAM group (P < 0.05, (Fig. 3)). Moreover, the ANOVA test showed a significant effect of treatment (F(5,12) = 11.22, p < 0.05), a significant effect of duration (F(6,22) = 21.18, p < 0.05) as well as a significant effect of week × treatment interaction (F(25,12) = 15.14, p < 0.05) on the sucrose preference in the CUMS groups. During the next 4 weeks of the CUMS trials, the SHAM/CUMS/solvent rats demonstrated a gradual decrease in sucrose preference as compared to the control non-CUMS SHAM group. The sucrose preference in long-term OVX rats from the 4th week till the end of the CUMS protocol progressively decreased as compared to the non-CUMS/CUMS SHAM rats (P < 0.05, (Fig. 3). VD3 (5.0 mg/kg, s.c.) increased sucrose preference in the 6th week in long-term OVX rats with CUMS when compared to the OVX group subjected to CUMS and treated with the solvent (P < 0.05, (Fig. 3)). Treatment with fluoxetine or paroxetine, as well as fluoxetine along with VD3 steadily elevated the sucrose preference of OVX/CUMS rats in the 5th week of CUMS protocol when compared to OVX/ CUMS/solvent and SHAM/CUMS/solvent rats (P < 0.05, (Fig. 3)). Combined administration of paroxetine and VD3 gradually increased the sucrose preference in OVX/CUMS rats in the 4th week when compared with OVX/ CUMS/ paroxetine/solvent and SHAM/CUMS/solvent rats (P < 0.05, (Fig. 3)).
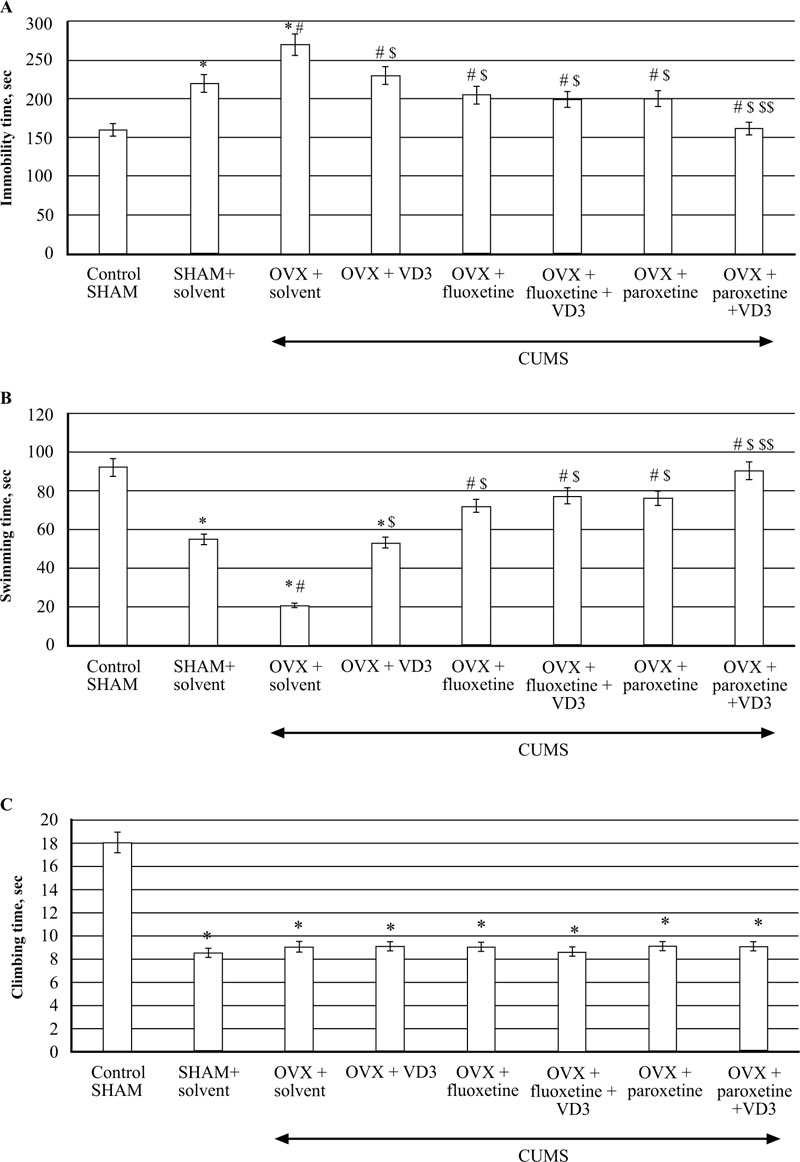
3.3. Combined Treatment (SSRIs with VD3) Corrects Depression-like Behaviour in FST of Long-term OVX Rats Exposed to CUMS.
CUMS produced a significant increase in immobility time and a decrease in swimming time in the long-term OVX rats compared to the non-CUMS/SHAM/CUMS rats (F(5,12) = 52.84 and F(5,12) = 68.89, respectively, P < 0.05, (Fig. 4A, B). VD3 (5.0 mg/kg, s.c.) decreased the immobility time and increased the swimming time of OVX/CUMS rats as compared to the OVX/CUMS/solvent group (P < 0.05, (Fig. 4A, B). Treatment with fluoxetine and paroxetine alone significantly reduced immobility time and increased swimming time in long-term OVX/CUMS rats as compared to the OVX/ CUMS/solvent and SHAM/CUMS rats (P < 0.05, (Fig. 4A, B). The values of immobility time and swimming time in OVX/CUMS/fluoxetine along with VD3 were similar to the values of OVX/CUMS/fluoxetine rats (P < 0.05, (Fig. 4A, B)). Paroxetine in combination with VD3 improved immobility time and swimming time in long-term OVX rats as compared to the OVX/CUMS/paroxetine/solvent and SHAM/CUMS/ solvent rats (P < 0.05, Fig. (4A, B). We found no difference in the climbing time in all the experimental groups compared to the OVX/SHAM/CUMS groups (P > 0.05, Fig. (4C)).
3.4. Combined Treatment (SSRIs with VD3) Changes Behaviour in OFT of Long-term OVX Rats Exposed to CUMS
After 6 weeks, the number of total crossings, crossings in the central squares, time spent in the central squares, and rearings of SHAM/CUMS rats significantly decreased compared to the non-CUMS SHAM group (F(5,12) = 64.43, P < 0.05, (Fig. 5A-D).
The number of total crossings, crossings in the central squares, time spent in the central squares, and rearings of long-term OVX rats with CUMS significantly decreased compared to the non-CUMS/CUMS SHAM groups (P < 0.05, (Fig. 5A-D)). Administration of VD3 enhanced the number of total crossings, crossings in the central squares, time spent in the central squares, and rearings of long-term OVX/CUMS rats compared to OVX/SHAM/CUMS groups (P < 0.05, (Fig. 5A-D)). Treatment with fluoxetine or paroxetine alone increased the number of total crossings, crossings in the central squares, time spent in the central squares, and rearings of long-term OVX/CUMS rats compared to the OVX/CUMS/solvent rats and SHAM/CUMS rats similarly to the OVX/CUMS rats treated with VD3 (P < 0.05, (Fig. 5A-D)). The crossings in the central squares and the time spent in the central squares of long-term OVX/CUMS rats after co-administration with fluoxetine or paroxetine in a combination with VD3 were markedly enhanced compared to OVX/CUMS/solvent/ fluoxetine/ paroxetine rats and SHAM/CUMS rats.
Following six months of CUMS protocol, we observed no statistically significant differences for grooming activities between all the experimental groups of rats in the OFT (F(5,12) = 0.82, P > 0.05, (Fig. 5E).

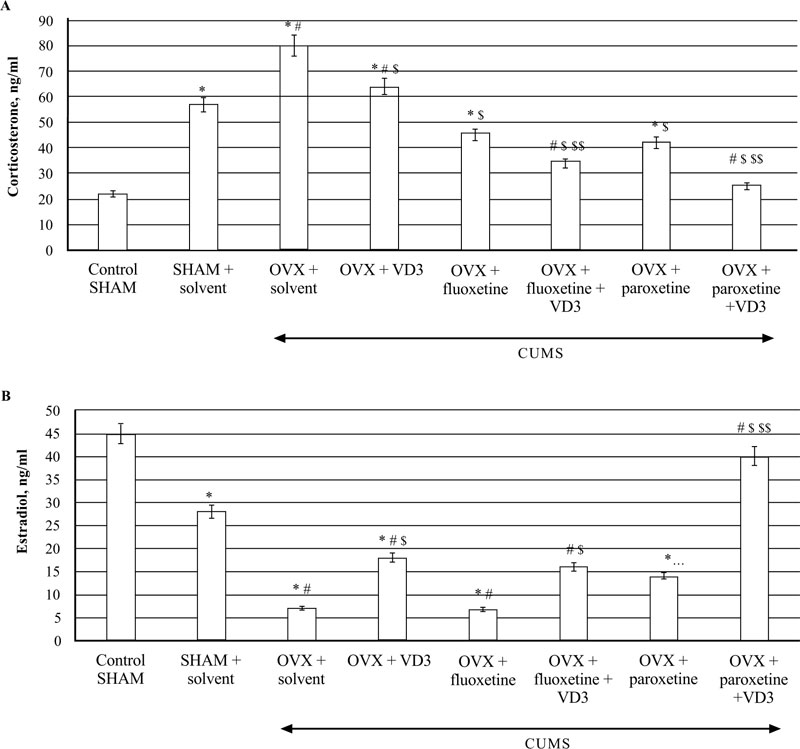
3.5. Combined Treatment (SSRIs with VD3) Alters Serum Corticosterone and Estradiol Levels in Long-term OVX rats Exposed to CUMS
The ELISA assay revealed that CUMS significantly increased CS concentrations in the blood of SHAM rats compared to the non-CUMS (control) female rats (P < 0.05, (Fig. 6A)). The serum CS levels were elevated in the long-term OVX/CUMS rats compared to the non-CUMS/SHAM/CUMS groups (F(5,12) = 78.56 and F(5,12) = 56.22, respectively, P < 0.05, (Fig. 6A). Supplementation with VD3 decreased CS and increased E2 levels of long-term OVX/CUMS rats compared to the OVX/CUMS/solvent rats (P < 0.05, (Fig. 6A, B)). SSRIs significantly reduced serum CS levels in the long-term OVX rats exposed to CUMS as compared to the OVX/SHAM plus solvent rats subjected to CUMS (P < 0.05, (Fig. 6A)). SSRIs in a combination with VD3 completely restored the serum CS levels in the long-term OVX rats exposed to CUMS when compared to the OVX/SHAM/fluoxetine/paroxetine rats subjected to CUMS (P < 0.05, (Fig. 6A)). Fluoxetine did not change serum E2 levels in the long-term OVX rats exposed to CUMS in comparison with the OVX/SHAM plus solvent rats subjected to CUMS (P < 0.05, (Fig. 6B)). Co-administration of fluoxetine with VD3 significantly increased serum E2 levels in long-term OVX rats as compared to the OVX/CUMS/solvent and SHAM/CUMS/solvent rats (P < 0.05, (Fig. 6A, B)). Paroxetine alone enhanced serum E2 levels in long-term OVX rats when compared to OVX/CUMS/solvent and SHAM/ CUMS/solvent rats (P < 0.05, (Fig. 6B)). The combination of paroxetine and VD3 profoundly increased E2 levels in long-term OVX rats when compared to OVX/CUMS/ paroxetine/solvent and SHAM/ CUMS/solvent rats (P < 0.05, (Fig. 6B)).
3.6. Combined Treatment (SSRIs with VD3) Modulates Hippocampal NF-kB and Pro-inflammatory Cytokine Levels in Long-term OVX Rats Exposed to CUMS
CUMS significantly increased NF-kB/p65/p50 levels in the hippocampus of SHAM rats compared to the non-CUMS control female rats (P < 0.05, (Fig. 7A)). CUMS resulted in a decrease of NF-kB/p50/p65 contents in long-term OVX rats compared to non-CUMS/CUMS SHAM rats (F(5,12) = 28.44 and F(5,12) = 26.04, P < 0.05, (Fig. 7A, B)). VD3 (5.0 mg/kg, s.c.) supplementation reversed NF-kB/p50/p65 levels in long-term OVX/CUMS rats compared to the OVX/CUMS/solvent rats (P < 0.05, (Fig. 7A, B)). No significant differences of fluoxetine or paroxetine were found on hippocampal NF-kB/p50/p65 levels of the long-term OVX rats exposed to CUMS when compared to the long-term OVX with CUMS rats treated with solvent (P > 0.05; (Fig. 7A, B)). The combination of SSRIs with VD3 significantly decreased NF-kB/p50/p65 concentrations in the hippocampus in long-term OVX/CUMS rats when compared to the OVX/CUMS/solvent rats (P < 0.05, (Fig. 7A, B)).
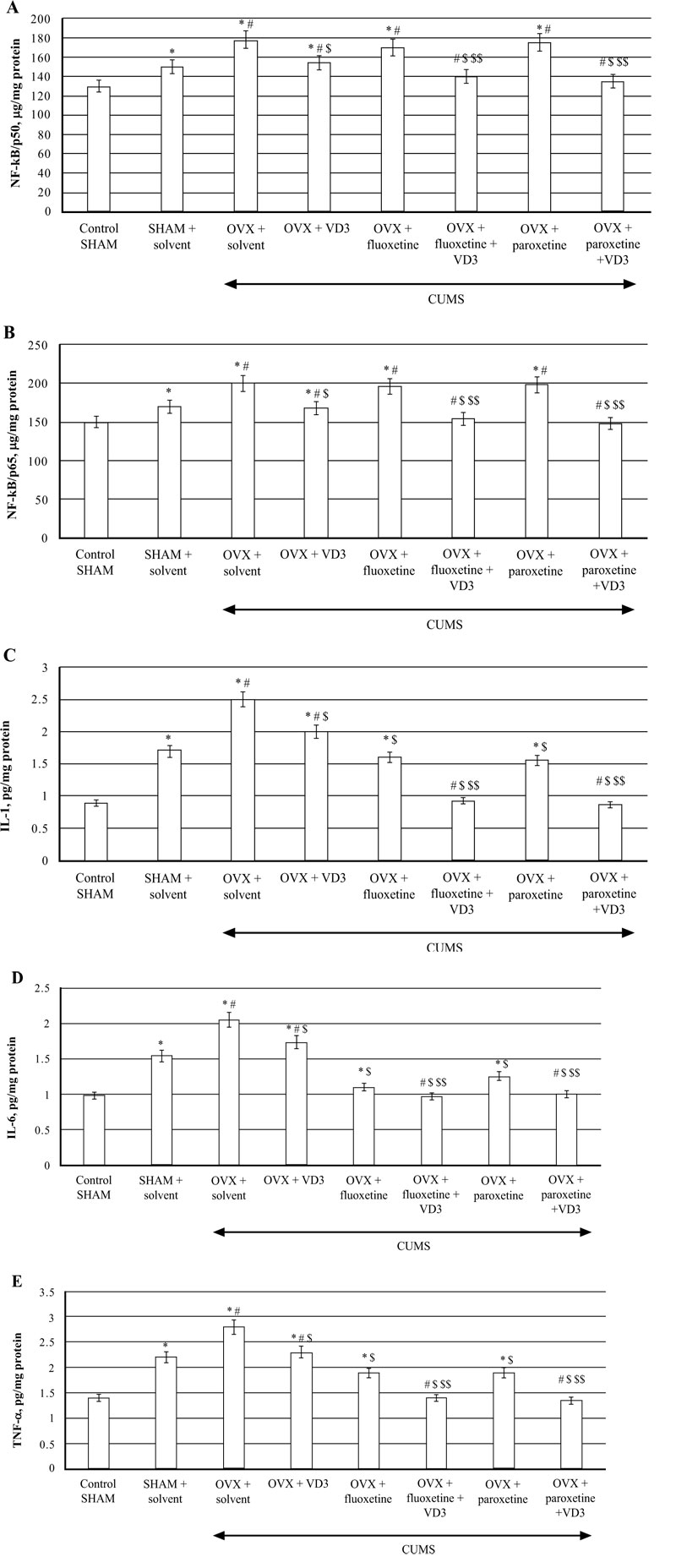
TNF-a, IL-1β, IL-6 concentrations in the hippocampus of SHAM rats were significantly increased compared to the non-CUMS control female rats (P < 0.05, (Fig 7C-E)). CUMS elevated hippocampal TNF-a, IL-1β, IL-6 levels in the long-term OVX rats compared to the non-CUMS/CUMS SHAM rats (F(5,12) = 28.44, (F(5,12) = 16.13, (F(5,12) = 22.10, P < 0.05, (Fig. 7C-E)). VD3 (5.0 mg/kg, s.c.) restored TNF-a, IL-1β, IL-6 levels in long-term OVX/CUMS rats compared to the OVX/CUMS/solvent rats (P < 0.05, (Fig. 7C-E)). Fluoxetine and paroxetine induced a decrease of TNF-a, IL-1β, IL-6 concentrations in long-term OVX/CUMS rats compared to the OVX/CUMS/solvent rats (P < 0.05, (Fig. 7B-D)). Co-treatment with SSRIs along with VD3 completely restored TNF-a, IL-1β, IL-6 levels in long-term OVX rats when compared with OVX/CUMS/fluoxetine/paroxetine/solvent and SHAM/CUMS/ solvent rats (P < 0.05, (Fig. 7C-E)).
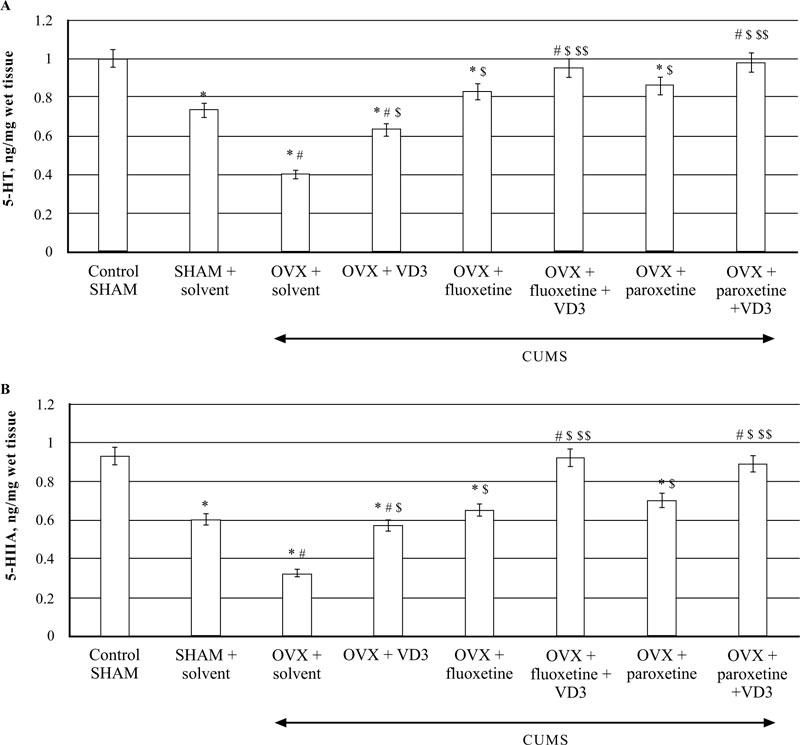
3.7. Combined Treatment (SSRIs with VD3) Restores Hippocampal 5-HT and 5-HIAA Levels in Long-term OVX rats Exposed to CUMS
The HPLC assay showed that CUMS decreased 5-HT levels in the hippocampus of SHAM rats as compared to non-CUMS control female rats (P < 0.05, (Fig. 8A)). In addition, the SHAM rats with CUMS showed a significantly enhanced 5-HT turnover in the hippocampus as compared to the non-CUMS control group. The post-hoc test indicated that long-term OVX rats with CUMS showed a more marked decrease of 5-HT concentrations in the hippocampus as compared to the non-CUMS/SHAM/CUMS rats (F(5,12) = 22.84, P < 0.05, (Fig. 8A). A significant increase in 5-HIAA contents was detected in the hippocampus of long-term OVX rats with CUMS as compared to the non-CUMS/SHAM/CUMS rats (F(5,12) = 34.56, P < 0.05, (Fig. 8B).
VD3 treatment (5.0 mg/kg, s.c.) restored 5-HT and 5-HIAA levels of OVX/CUMS rats as compared to the OVX/ CUMS/solvent group (P < 0.05, (Fig. 8A, B)). Treatment with SSRIs alone significantly elevated 5-HT and 5-HIAA levels in long-term OVX/CUMS rats as compared to the OVX/ CUMS/solvent and SHAM/CUMS rats (P < 0.05, (Fig. 8A, B)). SSRIs in a combination with VD3 resulted in normalization of 5-HT and 5-HIAA levels in long-term OVX rats as compared to the OVX/CUMS/fluoxetine/ paroxetine/ solvent and SHAM/CUMS/solvent rats (P < 0.05, (Fig. 8A, B)).
4. DISCUSSION
This study aimed to examine the antidepressant-like effects of combined treatment of different SSRIs (fluoxetine and paroxetine) along with VD3. A variety of behavioral tests were conducted on adult female rats with long-term lack of estrogen subjected to CUMS. The potential mechanism underlying the beneficial effects of a combination of SSRIs along with VD3 was examined. To examine if this combination of drugs could effectively diminish the behavioral impairments produced by CUMS, we explored proinflammatory cytokines, NF-kB/ p50/p65, and 5-HT/5-HIIA levels in the hippocampus. For the first time, proinflammatory cytokines, NF-kB/p50/p65, corticosterone/estradiol, and 5-HT/5-HIIA levels in the hippocampus were studied to evaluate the cooperative action of SSRIs and VD3 in adult long-term OVX rats exposed to CUMS.
The CUMS model is regarded as one of the most frequently experimented models of depression and anhedonia [47, 48]. Laboratory animals subjected to CUMS show patterns of behavioral alterations that are considered as experimental correlates of depression in humans [54, 55]. After being subjected to the CUMS protocols, SPT and FST were used to examine the anhedonia profile and behavioral despair in female rats, respectively. SPT is a standard test for the anhedonia-like state, which is postulated as a core sign of depression-like behavior in experimental models of depression [55]. FST is one of the most commonly used animal tests to assess the depression-like state and antidepressant-like effects of drugs in animals [56]. In this study, we examined the sucrose preference and body weight every week for 7 weeks to control the efficacy of CUMS protocol and concluded that 6 weeks of CUMS trials induced behavioral alterations in all experimental groups.
The results of this study show that SHAM/CUMS rats displayed decreased body weight, high anhedonia, depression-like, and anxiety-like symptoms in the SPT, FST, and OFT tests when compared with control non-CUMS animals. Moreover, SHAM/CUMS rats demonstrated elevated serum CS levels and hippocampal NF-kB/p50/p65, TNF-a, IL-1β, IL-6 levels, and reduced E2 and 5-HT/5-HIIA concentrations in the hippocampus as compared to the control non-CUMS animals.
As compared to SHAM/CUMS rats, the decrease in body weight, as well as symptoms of anhedonia and depression in adult long-term OVX rats were more obvious when assessed by SPT and FST, respectively. Long-term OVX rats exposed to CUMS exhibited decreased locomotor and rearing activities and increased anxiety-like behavior in the OFT. Our data agree with other findings indicating that long-term estrogen deprivation in female rodents subjected to a stress-related procedure results in a profound depression-like profile [57]. This study’s results demonstrated higher CS, NF-kB/p50/p65, TNF-a, IL-1β, and IL-6 levels in adult long-term OVX rats subjected to CUMS as compared to the SHAM/CUMS rats. Estrogen deficiency contributes to the impaired production of inflammatory cytokines in the whole organism [58]. These alterations in dysregulation in the production of inflammatory cytokines may extend to the brain and induce a state of neuroinflammation in the brain, which is directly associated with the development of affective-related disorders [15-21]. Our data are in an agreement with the experimental findings showing that ovariectomy elevates the production of proinflammatory cytokines [58]. The long-term OVX rats with CUMS demonstrated a decrease in 5-HT/5-HIIA levels in the hippocampus as compared to SHAM/CUMS rats. The current results confirm that CUMS produced marked behavioral, neuroendocrine, and neuroimmunological changes in adult SHAM or OVX rats with long-term estrogen deprivation (post-ovariectomy period of three months). These findings agree with our previous data and data reported in other studies [54, 55, 59].
After 28 days of therapy, we found that VD3 (5.0 mg/kg, s.c.) decreased the anhedonia and depression-like states in OVX/CUMS rats as published previously [42]. Moreover, decreased serum CS and hippocampal NF-kB/p50/p65, TNF-a, IL-1β, IL-6 levels, as well as increased E2 and 5-HT/5-HIIA concentrations in the hippocampus were also noted in OVX/CUMS animals treated with VD3 for 4 weeks as compared to the OVX/CUMS rats.
Treatment with the standard antidepressants, fluoxetine or paroxetine alone, induced a reduction of anhedonia and depression-like states in the SPT and FST from the 5th week of treatment, along with a decrease in the anxiety-like profile in the OFT of long-term OVX/CUMS rats as compared to OVX/SHAM/CUMS/solvent females. Simultaneously, long-term OVX/CUMS rats administered with tested SSRIs demonstrated a decrease of serum CS and hippocampal pro-inflammatory cytokine contents, and an increase of 5-HT/5-HIIA levels in the hippocampus as compared to OVX/ SHAM/CUMS/solvent rats. However, fluoxetine and paroxetine did not modify NF-kB/p50/p65 levels in the hippocampus of long-term OVX/CUMS rats submitted to CUMS. Our experimental data confirm that in a similar manner to other SSRIs, fluoxetine and paroxetine restore the functional activity of the HPA axis, 5-HT neurotransmission, and neuroinflammation in the brain, improving the depression-like state of OVX rats. Moreover, the data of the present study are in line with previous findings showing that paroxetine might induce an increase of E2 levels in females [45, 46].
Supplementation of VD3 to fluoxetine treatment failed to enhance the antidepressant-like effects of both drugs in long-term OVX rats with CUMS as compared to OVX/CUMS females treated with fluoxetine alone. However, this combination produced a profound decrease in serum CS concentrations and an increase of hippocampal 5-HT/5-HIIA levels in long-term OVX/CUMS rats in contrast to OVX/CUMS/fluoxetine rats. Moreover, only the application of fluoxetine with VD3 increased E2 levels and decreased NF-kB/p50/65 contents in long-term OVX/CUMS rats. A better comprehension of NF-κB-dependent mechanisms in the antidepressant action of SSRIs along VD3 requires further research. Co-administration of paroxetine with VD3 quickly and more effectively produced antianhedonic- and antidepressant-like effects in long-term OVX rats with CUMS as compared to treatment with paroxetine alone. In contrast to the behavioral effects of fluoxetine and VD3, long-term OVX/CUMS rats after 1 week of treatment with paroxetine and VD3 demonstrated a marked decrease in anhedonia and depression-like symptoms. Furthermore, co-treatment with paroxetine and VD3 produced synergic effects in decreasing serum CS and E2 levels as well as hippocampal pro-inflammatory cytokines contents.
In addition, enhancement of 5-HT/5-HIIA concentrations in the hippocampus was registered in long-term OVX female rats exposed to CUMS.
Interestingly, fluoxetine or paroxetine administered with VD3 cooperatively increased the total locomotor activity, rearings, the number of crossings, and time spent in central squares in the OFT in long-term OVX/CUMS rats as compared to in OVX/CUMS rats treated with fluoxetine or paroxetine alone. The number of crossings and time spent in central squares in the OFT are often interpreted as indicators of fear or anxiety in laboratory animals. Increases in these parameters can be induced by diazepam and other anxiolytics [60, 61]. All of the used drugs in the current study increased the number of crossings and time spent in central squares in the OFT in long-term OVX/CUMS rats. These results led us to conclude that fluoxetine or paroxetine administered with VD3 might similarly affect the anxiety-like profile in long-term OVX/CUMS rats as compared to its different effects on depression-like behavior in the FST. Based on these data, our next study will aim to assess exploratory activity and anxiety-like behavior in long-term OVX rats exposed to CUMS treated with fluoxetine and paroxetine along with VD3 using more specific tests for anxiety.
In conclusion, the synergistic effect of co-administration of paroxetine (10.0 mg/kg, i.p.) and VD3 (5.0 mg/kg, s.c.) completely abolished the CUMS-induced behavioral, neurohormonal, and neuroimmunological impairments in long-term OVX/CUMS rats. The supplementation of VD3 to fluoxetine failed to enhance the efficacy of this SSRI in long-term OVX/CUMS rats. However, it is important to note that co-treatment with fluoxetine and VD3 induces an increase in serum E2 concentrations and NF-kB/p50/p65 levels in the hippocampus of long-term OVX/CUMS rats. Therefore, it can be inferred that the efficacy of a combination of SSRIs with VD3 depends upon the SSIR used. This is why clinical and preclinical findings regarding the efficacy of a combination of SSRIs with VD3 are highly debated. [31, 62].
VD3 deficiency has been proven to impact the pathogenesis of various diseases, including mood disorders [26-29]. Moreover, VD3 is involved in the control of NF-kB signaling, 5-HT neurotransmission, and neuroinflammation in the brain [32-35]. Low VD3 levels are found in the majority of postmenopausal women [36-38]. Therefore, VD3 supplementation may be useful in treating mood disturbances in postmenopausal women with a low level of VD3. However, the exact role of VD3 supplementation in the prevention and treatment of mood disorders associated with menopausal consequences has not been completely identified. On the other hand, estrogen deficiency, like VD3 deficiency, also play a part in the mechanisms of affective-related disorders by modulating 5-HT system and pro-inflammatory cytokine levels in the brain [26, 27]. Such a complex interaction between estrogen and VD3 creates a neurobiological basis for their participation in similar behavioral disorders. Moreover, several investigations on the effects of VD3 on the brain have documented that VD3 can alter the synthesis of steroid hormones, including estrogen [63-66].
NF-κB is activated by different trigger factors stimulated by neuroinflammation [24, 25]. The up-regulation of NF-kB activity has been found in the experimental stress model [24, 25]. Pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α, are strongly involved in the pathophysiology of depression [17-20]. Some reports showed increased pro-inflammatory cytokine levels in patients with depression [18-21]. On the other hand, pro-inflammatory cytokines modulate the monoamines' functional activity and turnover of 5-HT in the brain [67-70]. Therefore, we believe that the beneficial effects of paroxetine and VD3 in the long-term OVX/CUMS rats are associated with the cooperative effects of these drugs on serum CS and E2 levels, and hippocampal TNF-a, IL-1β, IL-6, and 5-HT/5-HIIA concentrations.
Most of the current SSRIs exert an antidepressant-like effect by enhancing the 5-HT and/or other monoamines levels [5-9]. 5-HT is the main neurotransmitter that controls normal hippocampal neurogenesis [6, 7]. Therefore, we can assume that the antidepressant-like activity of SSRIs along with VD3 in long-term OVX rats subjected to CUMS is associated with complex positive effects of paroxetine and VD3 on depression-like behavior in CUMS model via restoration of 5-HT, as well as NF-kB/p50/p65 and pro-inflammatory cytokine levels in the brain. Further research is needed to clarify this hypothesis.
Among different classes of antidepressants, SSRIs are the most extensively used pharmacological drugs to treat affective-related profiles in perimenopausal and postmenopausal women [1-6]. The combined application of drugs is often used to achieve more considerable and faster therapeutic responses in the treatment of depression [71]. Therefore, VD3 supplementation to SSRIs may be effective in treating affective-related disturbances among hypoestrogenic females with VD3 deficiencies or insufficiencies. However, more research on this topic is still required since the exact role of VD3 in affective-related disorders associated with hormonal changes in women has not been completely understood.
The findings of this study provide experimental evidence regarding the beneficial effects of co-treatment with paroxetine along with VD3 in a CUMS model, producing cooperative antianhedonic- and antidepressant-like effects in the long-term OVX adult rats. Further research should explore the precise mechanism behind paroxetine and VD3 co-treatment in long-term OVX rats subjected to CUMS.
CONCLUSION
In conclusion, the results of this study suggest that combined treatment with fluoxetine (10.0 mg/kg, i.p.) or paroxetine (10.0 mg/kg, i.p.) along with VD3 (5.0 mg/kg, s.c.) produces distinct effects on depression-like behavior in long-term OVX rats exposed to CUMS. Co-administration of fluoxetine (10.0 mg/kg, i.p.) along with VD3 (5.0 mg/kg, s.c.) did not facilitate the effectiveness of fluoxetine in long-term OVX rats submitted to CUMS. The application of paroxetine (10.0 mg/kg, i.p.) along with VD3 (5.0 mg/kg, s.c.) releases faster and more considerable antianhedonic- and antidepressant-like effects in long-term OVX rats with CUMS than treatment with paroxetine alone. Our data indicate that the co-administration of paroxetine and VD3 may attenuate stress-induced CS/E2 and proinflammatory cytokine/NF-kB/5-HT alterations in the hippocampus of long-term OVX rats exposed to CUMS. This is the first report of the application of these drugs in combination to relieve anhedonia and depression-like state in long-term OVX rats exposed to CUMS. Our findings suggest that paroxetine and VD3 may be a therapeutic alternative to ameliorate the depression associated with menopause in women who have VD3 deficiency. This work provides translational information for the development of effective therapies for menopausal mood disorders in the future.
ETHICAL APPROVAL AND CONSENT TO PARTICIPATE
The study is approved by the Animal Care Committee of the I.P. Pavlov Institute of Physiology, Russian Academy of Sciences, Russian Federation, protocol 1095/1/25.06.2012.
HUMAN AND ANIMAL RIGHTS
No humans were used in this study. The current experiments on female rats were in accordance with the guidelines of National Institute of Health (National Research Council, Publication No. 85-23, revised in 1996), and the Animal Welfare Assurance Renewal for the I.P. Pavlov Institute of Physiology.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of this article is available at https://grant.rscf.ru upon the request.
FUNDING
The present study was supported by the Russian Science Foundation (RSF), research project No. 16-15-10053 (extension).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


