All published articles of this journal are available on ScienceDirect.
Human Brain Disorders: A Review
Abstract
Background:
Due to the stressful life, brain disorders are considered as a significant global healthcare problem. It has generated a great need for continuous research for understanding brain structure as well as functions in context to health and diseases.
Scope and Approach:
The structure and functions of the brain were questioned and studied since Ancient Greek times and led to the compilation of enormous information on the subject globally. With the advent of new technology, the researchers are able to discover the causes of brain diseases/disorders.
Conclusion:
In the present review, we have compiled various diseases and disorders related to the brain, along with their symptoms and the treatment strategies.
1. INTRODUCTION
The brain is the command center of the Central Nervous System (CNS) made up of a large mass of nerve cells, protected in the skull [1-3]. It has three main parts i.e. the cerebrum, the brainstem, and the cerebellum. It controls the intellectual activities of the body, like processing, integrating, and coordinating the information received from the sensory organs. It is a jelly-like mass of tissue, weighing about 1.4 kg, and containing 86 billion nerve cells [4-7]. The cerebrum is connected to the brainstem, which, on the other end, connects to the spinal cord. The brainstem consists of three parts, namely the midbrain, the pons, and the medulla oblongata. Underneath the cerebral cortex, there are several brain structures, namely the thalamus, the pineal gland, the hypothalamus, the pituitary gland, the amygdala and the hippocampus. The cross-section of each cerebral hemisphere shows a ventricle cavity where the cerebrospinal fluid is produced and circulated. Below the corpus callosum is the septum pellucidum, a membrane that separates the lateral ventricles [8, 9]. The cerebrum is the largest part of the human brain. It is divided into two cerebral hemispheres, having two-third of the total weight of the brain. One hemisphere is functionally dominant and controls language and speech. The other hemisphere interprets visual and spatial information. The two hemispheres of the human brain, the left and right, are connected together by a bundle of nerve fibers called the corpus callosum. Each hemisphere is divided into four lobes, namely the frontal lobe, temporal lobe, parietal lobe, and occipital lobe [10-13]. The frontal lobe controls cognitive functions and voluntary movements or activities such as emotional expression, problem-solving, memory, language, judgment, and sexual behaviors [14]. The temporal lobe controls the primary auditory perceptions such as hearing, and contains the primary auditory cortex, which receives the sensory information from the ears and secondary areas, and process information into meaningful terms which are expressed in the form of speech and words [15]. The parietal lobe processes information about temperature, taste, touch, and movement [14]. The occipital lobe is primarily responsible for vision [16].
2. BRAIN CELLS
The brain cells are referred as neurons and the supporting non-neuron cells are called glial cells. The average adult human brain contains approximately 86 billion neurons. It was suggested by many researchers that both neurons and glial cells are necessary for the proper functioning of the brain. Neurons in the brain are responsible for sending and receiving electrical and biochemical signals [17]. The neuron is made up of three basic parts: the cell body or soma, branching dendrites, and the axon. They are building blocks of the brain and transmit information to other neurons, muscles and tissues throughout the body.
Neurons help to think, feel, move, and comprehend the world around us. Glia cells are also very important cells of the nervous system. The name “glia” is the Latin word for “glue” [18]. The glial cells actively participate in brain signaling and are necessary for the healthy functioning of neurons. There are many types of glial cells in the brain. The three important types of glial cell are Oligodendrocytes: which help in insulating the axons and to pass the electrical signals properly at incredible speed over long distances; Microglia: also known as immune cells of the CNS, move around within the brain and constantly communicate with other glia cells; Astrocytes: These are star-shaped cells supporting to the Blood-Brain Barrier (BBB), provide nutrients to the neurons, repair the nervous tissue, and facilitate neurotransmission [19-21].
3. BLOOD BRAIN BARRIER (BBB)
Blood vessels are critical in delivering oxygen and nutrients to all the tissues and organs throughout the body. The blood vessels system of CNS is unique, constituting the BBB, which allow these vessels to tightly regulate the movement of ions, molecules, and cells between the blood and the brain [22]. The purpose of the BBB is to protect it against circulating toxins or pathogens, while at the same time allowing the vital nutrients to reach the brain. BBB is formed by the brain capillary endothelium [23]. It also maintains the level of hormones, nutrients and water in the finely tuned brain environment [24]. The delivery of therapeutic agents to the specific regions of the brain is a major challenge in the treatment of almost all types of brain disorders. The BBB hampers the delivery of many potentially important diagnostic and therapeutic agents to the brain. Therapeutic molecules and antibodies that might otherwise be effective in therapy do not cross the BBB in sufficient amount [25].
4. BRAIN DISORDERS
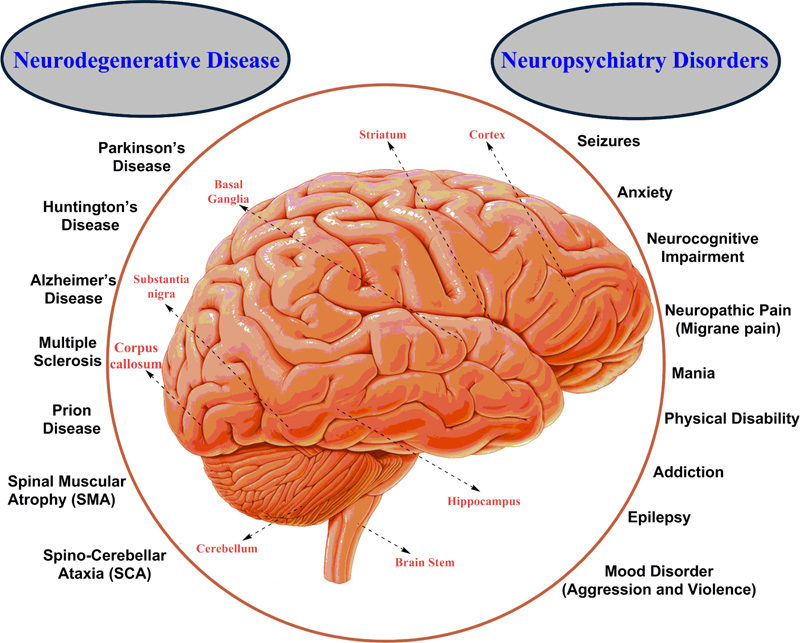
Any deformities, dysfunction and disease condition in the brain affect the whole body. The brain is susceptible to neuronal disease and neurons or tissue infection. Damage can be caused by trauma (psychiatric condition), or a loss of blood supply (accidental or environmental factors) known as a stroke. In brain injury, the degeneration of brain cells occurs [26, 27]. It depends upon a wide range of internal as well as external factors. Brain damage due to trauma is induced by personal factors or mentally unstable conditions, while neurotoxicity refers to chemically induced neuronal damage [26]. Broadly human brain disorders are divided into two categories namely, Neurodegenerative diseases and Neuropsychiatric disorders (Fig. 1). Both are tough to understand and incurable, but there are medicines, surgery and physical therapies applied for the treatment or to suppress the symptoms of the diseases (Table 1) [28-124].
| Category | S.No. |
Type of Diseases/ Disorders |
Parts of the Brain affected | Symptom(s) | Treatment(s) | Reference(s) |
| Autoimmune Diseases | 1. | Autoimmune encephalitis | Brain cells | Impaired memory and cognition, abnormal movements, seizures, and problems with balance, speech, or vision, psychosis, aggression, inappropriate sexual behavior, panic attacks, compulsive behaviors and euphoria or fear. | Intravenous immunosuppressive therapy and tumor removal. | [28,29] |
| 2. | Autoimmune epilepsy |
Brain cells | Recurrent and uncontrolled seizures. | Corticosteroids in addition to intravenous immunoglobulin or plasma exchange. | [30,31] | |
| 3. | Central nervous system vasculitis | Inflammation of blood vessels in the Brain | Headaches, strokes or transient ischemic attacks, forgetfulness or confusion weakness, problems with eyesight, seizures and encephalopathy (swelling of the brain). |
High-dose steroids such as prednisone, in combination with cyclophosphamide. | [32,33] | |
| 4. | Hashimoto’s encephalopathy | Lymphocytic vasculitis of venules and veins in the brain-stem and a diffuse gliosis involving gray matter or white matter | Personality changes, aggression, delusional behavior, concentration and memory problems, coma, disorientation, headaches, jerks in the muscles, lack of coordination, partial paralysis on the right side, psychosis, seizures (60% cases), sleep abnormalities, speech problems and tremors. | Corticosteroids | [34,35] | |
| 5. | Neuromyelitis optica | Eye nerves and spinal cord (myelin) | Blindness in one or both eyes, weakness or paralysis in the legs or arms, painful spasms, loss of sensation, uncontrollable vomiting and hiccups, and bladder or bowel dysfunction from spinal cord damage. | Intravenous corticosteroids and low doses of carbamazepine. | [36,37] | |
| 6. | Optic neuritis | Fatty coating (myelin) and optic nerve is inflamed. | Vision gets dim or blurry and loss of colour vision. | Intravenous immune globulin and vitamin B12. | [38, 39] | |
| 7. | Neurosarcoidosis or neurosarcoid | Facial and cranial nerves, hypothalamus | Bell’s palsy, leading to one-sided weakness of the facial muscles, double vision, hearing loss, headaches, speech problems, irritability, memory loss, changes in mood, dementia, hallucinations and seizures. | Corticosteroids, immunosuppressive medication, pain medication, radiation therapy, occupational and physical therapy. | [40, 41] | |
| 8. | Neuro-Behcet’s disease | Ventral brain stem | Fever, headache, genital ulcers, genital scars and skin lesions. | Azathioprine or methotrexate and corticosteroids. For high risk of patients intravenous cyclophosphamide with corticosteroids. | [42,43] | |
| 9. | Cerebral lupus or Systemic Lupus Erythematosus (SLE) | Brain cells | Fatigue, fever, joint pain, stiffness and swelling, butterfly-shaped rash on the face, skin lesions, fingers toes turn white or blue when exposed to cold or during stress, shortness of breath, chest pain and dry eyes. | Nonsteroidal anti-inflammatory drugs (NSAIDs) such as naproxen sodium (Aleve) and ibuprofen, antimalarial drugs such as hydroxychloroquine (plaquenil), corticosteroids prednisone, Immunosuppressants azathioprine (imuran, azasan), mycophenolate mofetil and methotrexate and rituxan. |
[44,45] | |
| 10. | Autism or Autism Spectrum Disorder (ASD) | Hippocampus, amygdala, lobes of the cerebrum ventricles and caudate nucleus. | Adoption of unusual speech patterns, such as using a robot-like tone, avoiding eye contact with others, not able to responding to their name, late development of speech skills, having difficulty with maintaining conversation, frequently repeating phrases, apparent difficulty in understanding feelings and expressing their own. | Behavior and communication treatments like play therapy, behavioral therapies, sensory therapies, occupational therapy, speech therapy, and only one medicine i.e. risperidone (risperdal). | [46, 47] | |
| Dementia | 1. | Fronto-temporal dementia | Degeneration of the temporal and frontal lobes. | Poor judgment, loss of empathy, socially inappropriate behavior, lack of inhibition, repetitive compulsive behavior, inability to concentrate or plan; frequent abrupt mood changes, speech difficulties. | Antidepressant drugs and Antipsychotic drugs. | [48, 49] |
| 2. | Dementia with Lewy bodies | Clumps of a protein in the cortex. | Sleep disturbances, hallucination, imbalance, movement difficulties. | Cholinesterase inhibitors, carbidopa and levodopa (sinemet, rytary, duopa). | [50, 51] | |
| 3. | Vascular dementia | Blocking of blood vessels. | Problems with short-term memory, laughing or crying at inappropriate times, trouble in concentrating, planning, or trouble in managing money, inability to follow instructions, loss of bladder or bowel control and hallucinations or delusions. | High blood pressure under control through exercise, diet and medication. | [52, 53] | |
| 4. | Alzheimer’s Disease (AD) | Destroy neurons and their connections in parts of the brain and affects areas in the cerebral cortex (plaque accumulation of abnormally folded amyloid β-protein and tau protein in the brain). | Having less energy, less interest in work and social activities, loss of memory, like forgetting conversations and events that just happened, language problems, coordination problems, mood swings and depression. | Donepezil (Aricept), Rivastigmine (Exelon), Tacrine (Cognex) | [54, 55] | |
| Brain Infections | 1. | Meningitis | Inflammation of the meninges, brain and spinal cord. | Decreased appetite, irritability, sleepiness, lethargicity, fever, sensitivity to bright light, sleepiness, nausea, vomiting, confusion and disorientation. | Antibiotics given directly into a vein, fluids given directly into a vein to prevent dehydration, oxygen through a face mask if there are any breathing difficulties, steroid medication to reduce any swelling around the brain. | [56, 57] |
| 2. | Encephalitis | Temporal lobe, frontal lobe | Headache, fever, aches in muscles or joints, fatigue or weakness, confusion, agitation or hallucinations, seizures, loss of sensation or paralysis in certain areas of the face or body, muscle weakness, problems with speech or hearing and loss of consciousness. | Corticosteroids, acyclovir (Zovirax) and ganciclovir (Cytovene). | [58, 59] | |
| 3. | Brainabscess | Fungal and viral infection in brain | Differences in mental processes, such as increased confusion, decreased responsiveness, and irritability, speech, sensation, decreased movement due to the loss of muscle function, changes in vision, changes in personality or behavior, vomiting, fever, chills, neck stiffness, especially when it occurs with fever and chills, sensitivity to light. | Antibiotic medications and surgery. | [60, 61] | |
| Movement Disorders | 1. | Ataxia | Cerebellum | Poor coordination, unsteady walk and a tendency to stumble, difficulty with fine motor tasks, such as eating, writing or buttoning a shirt, change in speech, involuntary back-and-forth eye movements (nystagmus) and difficulty in swallowing. | Occupational therapy, speech and language therapy, physical and mental exercise. | [62, 63] |
| 2. | Dystonia and Cervical dystonia | Basal ganglia | Dragging leg, cramping of the foot, involuntary pulling of the neck, uncontrollable blinking and speech difficulties. | Levodopa, procyclidine, hydrochloride, diazepam, lorazepam, clonazepam and baclofen | [64, 65] | |
| 3. | Chorea | Basal ganglia | Problem in speaking, swallowing, posture and gait. | Medication like Fluphenazine (Prolixin), Haloperidol (Haldol), Olanzapine (Zyprexa), Quetiapine (Seroquel), Risperidone (Risperdal). | [66, 67] | |
| 4. | Huntington’s Disease (HD) | Nerve cells and basal ganglia | Involuntary jerking or writhing movements, muscle problems, such as rigidity or muscle contracture, slow or abnormal eye movements, impaired gait, posture and balance, difficulty in speech or swallowing. | Medication like Tetrabenazine (Xenazine), antipsychotic drugs, such as Haloperidol (Haldol) and Chlorpromazinamantadine, Levetiracetam (Keppra, others) and Clonazepam (Klonopin). | [68, 69] | |
| 5. | Multiple System Atrophy (MSA) | Cerebellum, basal ganglia and brainstem | Impaired movement and coordination, such as unsteady gait and loss of balance, slurred, visual disturbances, difficulty swallowing (dysphagia) or chewing, constipation, agitated sleep due to “acting out” dreams, abnormal breathing at night, inability to achieve or maintain an erection, loss of libido, irregular heartbeat and difficulty in controlling emotions. | Corticosteroid Fludrocortisone, Epyridostigmine (Mestinon), Midodrine Levodopa and Carbidopa (Duopa, Sinemet). | [70, 71]. | |
| 6. | Myoclonus | Cerebral cortex, cerebellum and brainstem | Sudden, brief, involuntary, shock, variable in intensity and frequency, localized to one part of the body or all over the body, sometimes severe enough to interfere with eating, speaking or walking. | Tranquilizers like Clonazepam (Klonopin), anticonvulsants like Levetiracetam (Keppra, Roweepra, Spritam), Valproic acid (Depakene) and Primidone (Mysoline), Piracetam onabotulinumtoxin A (Botox) injections, deep brain stimulation (DBS). |
[72,73] | |
| 7. | Parkinson’s Disease (PD) | Nerve cells, basal ganglia and substantia nigra | Tremor, shaking hand or fingers, slowed movement (bradykinesia), rigid muscles, impaired posture and balance, loss of automatic movements, speech changes and writing changes. | Levodopa, the most effective carbidopa-levodopa infusion, dopamine agonists, MAO-B inhibitor, catechol O-methyltransferase (COMT) inhibitors, anticholinergics, amantadine, DBS. |
[74, 75] | |
| 8. | Progressive supra-nuclear palsy or Steele-Richardson- Olszewski syndrome |
Basal ganglia and the brain stem | Stiffness and awkward movements, problems with speech and swallowing, sensitivity to light, sleep disturbances, loss of interest in pleasurable activities, impulsive behavior, possibly including laughing or crying for no reason, difficulties with memory, reasoning, problem-solving and decision-making, depression and anxiety. | Physical and occupational therapy, medication like onabotulinum toxin type A (Botox), eyeglasses with bifocal or prism lenses. | [76, 77] | |
| 9. | Restless Legs Syndrome (RLS) | Cingulate cortex and cerebellum | Sensations after rest, worsening of symptoms in the evening and night, time leg twitching. | Ropinirole (Requip), Rotigotine (Neupro), Pramipexole (Mirapex), Gabapentin (Neurontin, Gralise), Gabapentin Enacarbil (Horizant), Pregabalin (Lyrica), Tramadol (Ultram, ConZip), Codeine, Oxycodone (Oxycontin, Roxicodone, others) and Hydrocodone (Hysingla ER, Zohydro ER), muscle relaxants and sleep medications. | [78, 79] | |
| 10. | Tardive dyskinesia | Striatum and basal ganglia | Tongue movements, lip smacking, lip puckering, pursing of the lips and excessive eye blinking | Medication like Deutetrabenazine (Austedo) Valbenazine (Ingrezza), Melatonin, Vitamin B6, Vitamin-E |
[80,81] | |
| 11. | Tourette syndrome | Basal ganglia | Eye blinking, head jerking, repeating observed movements, shoulder shrugging, stepping in a certain pattern, eye darting, obscene gesturing, nose twitching, bending or twisting, mouth movements and hopping. | Medications that block or lessen dopamine, botulinum (Botox) injections, ADHD medications, central adrenergic inhibitors anti-depressants, anti-seizure medications, behaviour therapy, psychotherapy and deep brain stimulation. | [82, 83] | |
| 12. | Wilson's disease | Brain and spinal cord | Fatigue, lack of appetite or abdominal pain, yellowing of the skin and the white of the eyes (jaundice), golden-brown eye discoloration (Kayser-Fleischer rings), fluid buildup in the legs or abdomen, problems with speech, swallowing or physical coordination and uncontrolled movements or muscle stiffness. | Medication like Penicillamine (Cuprimine, Depen), Trientine (Syprine), T-Zinc acetate (Galzin) and Surgery. | [84, 85] | |
| Neuromuscular Diseases | 1. | Amyotrophic Lateral Sclerosis (ALS) | Degeneration of nerve cells in the spinal cord and brain. | Twitching and cramping of muscles, loss of motor control in the hands and arms, impairment in the use of the arms and legs, tripping and falling, dropping things, persistent fatigue, uncontrollable periods of laughing or crying, slurred or thick speech and trouble in projecting the voice. | Physical, occupational, speech, respiratory, and nutritional therapies. | [86, 87] |
| 2. | Charcot-Marie-Tooth disease | Axon (myelin sheath) | Weakness of foot and lower leg muscles, foot deformities, difficulty in lifting foot while walking, loss of muscles around hands and feet, numbness, tingling, burning, or loss of temperature sensation in hands and feet, discomfort or pain in hands and feet. |
Physical therapy, occupational therapy, orthopedic devices, pain relieving medicines. | [88, 89] | |
| 3. | Multiple sclerosis | Brain, spinal cord and nerve cells | Blurred or double vision, red-green color distortion, pain and loss of vision because of swelling of the optic nerve (optic neuritis), trouble in walking, numbness, prickling, or pins and needles (paresthesia), muscle weakness in the arms and legs, trouble with coordination, fatigue, loss of feeling, speech problems, tremors, dizziness, hearing loss, bowel and bladder problems and depression. | Exercise programs that build muscle strength, endurance, and control. | [90, 91] | |
| 4. | Muscular dystrophy | Absence of protein dystrophin in neurons | Progressive muscle weakness and wasting (atrophy), waddling way of walking, difficulty in climbing stairs, repeated falling, curvature of the spine, wasting of thigh muscles, abnormal enlargement of the calves, trouble in breathing and swallowing and enlargement of the heart. | Physical therapy and exercise, Surgery and Prednisone. | [92, 93] | |
| 5. | Myasthenia gravis | Neurons | Visual problems including drooping eyelids (ptosis) and double vision (diplopia), muscle weakness and fatigue, weakness of the neck or limbs. | Anti-cholinesterase medicines, steroids, or medicines that suppress the immune system’s response, thymectomy and plasmapheresis | [94, 95] | |
| 6. | Myositis, including polymyositis and dermatomyositis | Bubbles in the brain | Muscle weakness, painful muscles, extreme tiredness, generally feeling unwell, shortness of breath. | Exercise and physiotherapy, steroid medication, Intravenous immunoglobulin therapy, Rituximab injection | [96, 97] | |
| 7. | Peripheral neuropathy | Nerves in the brain and brainstem | Gradual onset of numbness, jabbing, throbbing or burning pain, lack of coordination, muscle weakness, paralysis if motor nerves are affected. | Pain relievers, anti-seizure medications, topical treatments, antidepressants, therapies, transcutaneous electrical nerve stimulation (TENS). | [98, 99] | |
| 8. | Spinal muscular atrophy | Spinal cord and brain stem | Muscle weakness and twitching, difficulty in breathing and swallowing, changes in the shape of the limbs, spine, and chest due to muscle weakness, difficulty in standing and walking. | Drugs such as spinraza and zolgensma. | [100, 101] | |
| Seizure Disorders | 1. | Tonic-clonic seizures or Grand- mal seizure | Cerebellum, basal ganglia, brainstem and thalamus. | Loss of bowel and bladder control, unresponsiveness after convulsions, confusion, fatigue and severe headache. | Drugs like carbamazepine (Carbatrol, Tegretol), Phenytoin (Dilantin, Phenytek), valproic acid (Depakene), oxcarbazepine (Oxtellar, Trileptal), Lamotrigine (Lamictal), Gabapentin (Gralise, Neurontin), Topiramate (Topamax), Phenobarbital and Zonisamide (Zonegran) | [102, 103] |
| 2. | Atonic seizures | Alterations in brain function | A sudden loss of muscle strength, going limp and falling to the ground, if seated, the person’s head will appear to suddenly drop down, remaining conscious, drooping eyelids, head nods and jerking movements. | Ethosuximide (Zarontin) and Valproic acid (Depakene) | [104, 105] | |
| 3. | Myoclonic seizures | Temporal lobe | Sudden increases in muscle tone, sudden spasm, occasionally and falling asleep. | Anti-seizure medication, nerve stimulation, dietary therapy or surgery. | [106] | |
| 4. | Absence seizures or Petit mal seizures | Thalamus | Sudden stop in motion without falling, lip smacking, eyelid flutters, chewing motions, finger rubbing and small movements of both hands. | Ethosuximide (Zarontin), Valproic acid (Depakene) and Lamotrigine (Lamictal). | [107,108] | |
| Stroke Diseases | 1. | Trauma | Alterations in brain functioning. | Loss of consciousness for a few seconds to minutes, confused or disoriented state of mind, headache, nausea or vomiting, fatigue or drowsiness, problems with speech, difficulty in sleeping, dizziness and loss of balance. | Immediate emergency care, medication, surgery and rehabilitation. | [109, 110] |
| 2. | Tumors | Brain cells | Headache, nausea, vomiting, vision problems: such as blurred vision, double vision or loss of peripheral vision, gradual loss of sensation or movement in an arm or a leg, speech difficulties and hearing problems. | Medication, surgery, rehabilitation, minimally invasive scar less brain surgery, radiation therapy, chemotherapy and targeted drug therapy. | [111, 112] | |
| Mental Disorders | 1. | Anxiety disorders, including panic disorder, obsessive-compulsive disorder, and phobias | Amygdala | Fatigue, trouble in sleeping, muscle tension or muscle ache, trembling, feeling twitchy, nervousness, sweating, nausea, diarrhea and irritability. | Psychotherapy, drugs like antidepressants, buspirone, benzodiazepines are used. | [113, 114] |
| 2. | Post-traumatic stress disorder | Cingulate cortex and frontal gyrus | Negative thoughts, memory problems, difficulty in maintaining close relationships and emotionally numb. | Psychotherapy, cognitive therapy, eye movement desensitization and reprocessing (EMDR). Medications like anti-depressants and anti-anxiety (Prazosin). |
[115,116] | |
| 3. | Psychotic disorders, including schizophrenia | Medial frontal lobe | Delusions, hallucinations, disorganized thinking (speech), extremely disorganized or abnormal motor behavior, negative symptoms, sleep disturbances, irritability or depressed mood and lack of motivation. | Cornerstone, antipsychotic medications, aripiprazole (abilify), asenapine (saphris), brexpiprazole (rexulti), paliperidone (invega), quetiapine (seroquel), risperidone (risperdal), ziprasidone (geodon) and long-acting injectable antipsychotics. | [117, 118] | |
| 4. | Depression, bipolar disorder, and other mood disorders | Hippocampus | Increased activity, energy or agitation, exaggerated sense of well-being and self-confidence (euphoria), sleep disturbances, unusual talkativeness or making foolish investments. | Mood stabilizers, anti-psychotics, anti-depressants, antipsychotic and anti-anxiety medications. | [119, 120] | |
| 5. | Eating disorders | Brain cells | Abnormally low body weight, intense fear of gaining weight, repeatedly and persistently regurgitating food after eating. | Healthy eating, psychotherapy, family-based therapy (FBT), cognitive behavioral therapy (CBT), antidepressants and anti-anxiety medications. | [121, 122] | |
| 6. | Personality disorders | Amygdala, pre-frontal cortex | Aggression toward people and animals, destruction of property deceitfulness, theft, serious violation of rules, exploit others, poor or abusive relationships, being consistently irresponsible. | Psychotherapy | [123, 124] |
4.1. Neurodegenerative Diseases
Neurodegenerative Diseases are a composite form of disorders characterized by progressive loss of neurons, which hamper the function of the Central Nervous System (CNS) as well as Peripheral Nervous System (PNS). These diseases show their impact on both mental as well as and physical activity of the human body such as, movement, speech, memory, intelligence and coordination. The causes of these diseases are not specific due to the similarity in symptoms. Some common neurodegenerative diseases are Alzheimer’s Disease (AD), Parkinson’s Disease (PD), Prion disease, Huntington’s Disease (HD), Spino-Cerebellar Ataxia (SCA) and Spinal Muscular Atrophy (SMA) [125]. In AD, neuron death has been reported amygdala, cortex, and hippocampal region of the brain [126]; while in PD, substantia nigra pars compacta shows neuronal death that leads to the deficiency of dopamine [127].
Alzheimer’s Disease (AD) is the most prevalent and mainly affects the population of above 60 years. It was first described in 1906 by German Physician Alois Alzheimer on the basis of a medical case of Auguste D, a patient who showed symptoms of memory loss and some psychological changes like mood swing and unresponsive behavior. AD was named after German Doctor Alois Alzheimer by Emil Kraepelin, who was the colleague of Dr. Alzheimer. In 1910, he mentioned for the first time this disease as “Alzheimer’s disease” in his medical book named ‘Psychiatrie’ [128, 129]. It is charac- terized by progressive memory loss and cognitive impairments. Mainly two proteins are responsible for causing this disease i.e., beta-amyloid (β) and tau protein which show a high amount of accumulation in the brain. Both proteins may lead to the degeneration of neurons [126]. The pathological hallmark of AD includes, the presence of extracellular amyloid β plaques and formation of intracellular neurofibrillary tangles that leads to synaptic dysfunction, neuronal loss, and brain atrophy [130].
Parkinson’s Disease (PD) is the second most common neurodegenerative disease after AD. It is a progressive CNS disorder that affects mainly the movement of the body. It affects both motor as well as non-motor functions. The causes of PD are still unknown, but the involvement of both genetic and environmental factors considered to be responsible for the progression of the disease. It was described by Dr. James Parkinson (1817) in his monograph entitled “An Essay on the Shaking Palsy” [131, 132]. It is characterized by the loss of dopaminergic neurons and the accumulation of Lewy bodies in the substantia nigra pars compacta of the mid brain. Clinical symptoms mainly involves rigidity, akinesia, tremors, postural instability, and non-motor symptoms [133].
In 1920, Neurologists Hans Gerhard Creutzfeldt and Alfons Maria Jakob describe prion disease as a human neurological disorder. Prion disease is also known as Transmissible Spongiform Encephalopathies (TSEs) and is characterized as rare progressive neurodegenerative disorders which not only affect humans but also animals [134]. In this disease a normal cell surface glycoprotein (PrPC) is converted into conformational altered isoform i.e. (PrPSc) . PrPSC is responsible for the neurological disorder [135]. The most common form of prion disease that affects human is Creutzfeldt-Jakob Disease (CJD) and animal (cows) bovine spongiform encephalopathy (BSE or ‘mad cow’ disease). Prions are protein that are misfolded and also have the property to propagate [136, 137].
Huntington’s Disease (HD) is also known as Huntington’s chorea. It results due to the progressive degeneration of neuronal cells in the brain. George Huntington, described it in 1872 as a hereditary neurodegenerative disease [138]. It is an autosomal-dominant neurodegenerative disease which results from the unstable trinucleotide repetition of Cytosine-Adenine-Guanine (CAG). It is clinically characterized by involuntary movements, cognitive decline, and behavioral changes [139].
4.2. Neuropsychiatry Disorders/Conditions
Neuropsychiatry is a kind of disorder or conditions that deals with mental disruption which results due to the improper functioning of the brain. It is defined as mental disorders or disorders of the brain [140]. According to Berrios and Markova,, neuropsychiatry explains about brain having some lesions that disfigured the brain leading to mental disorder [141]. Neuropsychiatric disorders severely affect the well-being of a person with a negative impact on general health. It hampers the ability of learning (childhood) and inability of focusing or concentrating in work (adulthood) [141, 142]. They are complex and hard to understand because of the similarity in symptoms. Some common neuropsychiatric disorders are seizures, attention or cognitive deficit disorders, uncontrollable anger, migraine headaches, addictions, eating disorders, depression and anxiety. The person suffering from neuropsychiatry disorders shows changes in behavior i.e. aggression, violence, criminal activity, antisocial personality disorder, psychopathy, impulse control disorders and episodic dyscontrol [143, 144]. The causes of brain disorders are still unclear, but some genetic as well as environmental factors are responsible for the diseased condition. These disorders have a relatively high prevalence and show early onset (autism in childhood and schizophrenia in adulthood) [145, 146]. Table 1 summarizes the different categories of brain disorders with their symptoms and preventions.
5. CAUSES OF NEURODEGENERATIVE DISEASES AND NEUROPSYCHIATRY DISORDERS
The role of genes and the environment for the progression of neurological disease/disorders cannot be ignored. Any damage to the CNS leads to cell death which leads to the loss of function [147]. The brain disorders hampered the normal functioning of the brain and lead to the progressive decline or sudden complete loss of brain functions (sensory, motor, and cognitive) [148]. There are some neurodegenerative diseases which are characterized by the abnormal accumulation of the protein in the brain tissue i.e. Tau protein, β-amyloid (accumulation of plaques in the form of neurofibrillary tangles) in AD, misfolded Huntingtin protein in HD, aggregation of ubiquitinated proteins in ALS, α-synuclein accumulation in PD, and cell surface glycoprotein accumulation in prion disease [149-152]. Some studies have suggested that the mutation in genes leads to the accumulation of misfolded protein. Physical injury to the brain may lead to synaptic insufficiency, massive cell death and inflammation that may lead to temporary or permanent loss of various bodily actions like coordination in the movement (ataxias) and different cognitive functions like memory, learning, decision-making skills, talking and dementia. There is no permanent treatment for neurodegenerative diseases. In advanced stages of the diseases, DBS and cell transplantation therapies are used for controlling/reducing the physiological as well as cognitive deficits [153-155].
In AD, aggregation of β-amyloid protein accelerates the formation of neurofibrillary tangles which leads to synaptopathy in the form of glial inflammation and neuronal cell death in the cerebral cortex, sub-cortical regions, temporal lobes, parietal lobes, and cingulate gyrus in brain [156]. In PD the depletion of dopamine producing neurons in substantia nigra is accelerated by the intracellular accumulation of protein α-synuclein bound to ubiquitin complex. These protein aggregates form cytoplasmic inclusions in the form of Lewy bodies, which play a significant role in familial as well as sporadic cases of PD [133]. Similarly, HD is also caused by the intracellular accumulation of Huntingtin protein. The mutation in huntingtin (gene) results in the death of cells in the striatum region of the brain [138]. Multiple Sclerosis (MS) is a glial disorder. It involves massive damage to myelinated fibers through autoimmune reaction, causing axonal injury and further loss of neuronal communication mostly in the white matter tracts, the basal ganglia, and the brain stem [157]. It has been reported that the genetic factors responsible for AD ranges from 49-79% [156]. Similarly, for PD it ranges from 5-10% [156]. While, HD is considered as a pure genetic disorder which is caused by tri-nucleotide repeat expansions (CAG) nucleotides [138]. The whole genome sequence may help both researchers as well as clinicians to understand the genetic factors that play an important role in health, disease, and drug response [158]. The interplay of genetic and environmental factors that hampers the brain function is difficult to understand [159]. It was studied that in schizophrenia and bipolar disorders, genetic factors play a huge role in the development of disease. In a twin study, it ranges from 70-80%. Similarly, in depression, the genetic factors showed significant increment of 38% to 75% [160, 161]. In dementia cases, 1 in 4 person aged above 55 has a family history of dementia [162].
Dementia is an umbrella term used to demonstrate a group of symptoms in neurodegenerative diseases. The late stages of the neurodegenerative diseases lead to significant cognitive dysfunctions that are enough to affect a routine life. The symptoms of Dementia shows deficits in memory and learning, impairment in visual, loss of attentive function, as well as behavioral disturbances [162]. Epigenetic factors also play an important role in aggravating the symptoms or enhancing the disease like symptoms. Some metals have been reported to contribute for the progression of AD and PD i.e. lead (Pb), Mercury (Hg), Arsenic (As), Cadmium (Cd), Almuniun [163, 164]. Pesticides also play an important role in neurological disorders like Paraquat (PQ) and 1-methy l-4 phenyl l-1, 2, 3, 6-tetrahydropyridine (MPTP) [165]. Rotenone, like Trichloroethylene (TCE) and toxic nanoparticles have also been shown to cause neuronal cell damage and improper functioning of the CNS [166, 167].
6. DATABASE FOR NEURODEGENERATIVE DISEASES
Due to the complex pathophysiology and overlapping of symptoms in various neurodegenerative diseases it is the need of the hour to build a database for neurodegenerative diseases. Researchers have developed an online web database (DND: Database of neurodegenerative disorders) that contains more than 100 neuro related disease concepts having the information of all related genes, their products, pathophysiological pathways and treatment strategies [168]. It provides enormous data related to almost every aspect of neurodegenerative disorders for better understanding of molecular and genetic pathways involved in the progression as well as treatment of the disease [168]. Researchers from University of Pennsyl- vania, with the help of a consortium of Penn investigators, have developed a novel Integrated Neurodegenerative Disease Database (INDD) for AD, PD, ALS and Fronto-temporal lobar degeneration [169]. This database (Penn INDD) has the ability to query multiple database tables from a single console with a high degree of precision and reliability. It is also useful for comparative studies of various neurodegenerative diseases [169]. Kandale et al. [170] have included 18 diseases in Integrated Database of Neurodegenerative Diseases (IDND). They have prepared IDND by using three separate databases i.e. UniProt kB (protein information), kEGG (Pathway), PubMed (disease articles).
PD Gene is another dedicated online resource that comprehensively collects and meta-analyzes all published studies in the related field [171]. This database help researchers to decipher the genetic architecture underlying PD susce- ptibility. With the help of this database ITGA8 was identified as a novel potential PD risk locus [171].
Alz Data is related to the most prevalent and rapidly increasing neurodegenerative disorder i.e., Alzheimer’s disease. This database includes: (i) High throughput omic data e.g. Genomics (GWAS and Whole Exome Sequencing), Transcriptomes, Proteomics and Functional genomics; (ii) High Confident functional data, e.g. neuroimaging screening, population base longitudinal studies and transgenic mouse phenotyping [172].
Schizophrenia is one of the common psychiatric disorders having heritability of about 80% [173]. The genetic and molecular studies carried out on Schizophrenia have been deposited in database i.e. SZDB. Recently, a new version of a comprehensive database for Schizophrenic research has been launched i.e. SZDB 2.0 (www.szdb.org) [174]. The new version includes Genomes Wide Association Study (GWAS), polygenic risk score calculator, genetic and gene expression studies, copy number variations, gene expression Quantitative Trait Loci (eQTL), transcript QTL, methylation QTL and protein-protein interaction data [174]. This database will definetly provide a good platform for the enhancement of the Schizophrenia research.
BD gene is another database that was designed to address the genetic complexities of Bipolar Disorder (BD) and its overlapping with Schizophrenia as well as Major Depressive Disorder (MDD) [173]. It is freely available for the researchers. It provides not only a detailed review of research but also provides details for high confidence candidate genes and pathways for better understanding the pathology of the disease [173].
7. MODELS TO STUDY BRAIN DISORDERS
A number of animal model such as Caenorhabditis elegans, Drosophila melanogaster (fruitfly), Musca domestica (house fly), Danio rerio (zebra fish), pig and monkeys are used for understanding molecular pathways involved in various categories of brain disorders/diseases [175]. Cell lines are also used to explore the molecular pathways involved in the progression of brain diseases/disorders. Recent studies have revealed a great similarity between monkey and human brain (both in structure as well as in organization). It is expected that it will help a lot in understanding human brain diseases/disorders [176]. However, the selection of the model depends on the nature of the biological questions to be answered [176]. C. elegans has been used as a model organism for studying various aspects of neurodegenerative diseases like PD [177], AD [178], and HD [179], due to the conserved counterparts in C. elegans. The improved transgenic technology has led Drosophila as a model for number of neurodegenerative disorders such as AD, taupathies, PD, amyotrophic sclerosis, hereditary spastic paraplegia and various polyglutamine diseases [180-182]. Zebra fish genes and their human homologues have conserved functions with respect to the etiology of neurodegenerative diseases including PD, HD and AD [183]. The larvae of Zebrafish display neuro-pathological and behavioral phenotypes that are quantifiable and comparable to humans [184]. By using genetic manipulation techniques, transgenic mice and rats have been developed to understand the pathophysiology of autism, Fragile X syndrome (FXS) and other neuropsychiatric disorders [185-187]. The cerebral cortex of pig, unlike that of mice or rat, has cerebral convolution (gyri and sulci) similar to human neo-cortex and thus is expected to yield high translational value [188]. The use of pig in neuroscience for the modeling of human brain disorders has been extensively reviewed by Lind et al. [189]. Pigs are more similar to humans than mice in anatomy, physiology and genome, hence genetically modified pigs are also being used to study various neurological disorders [190]. Neurological disorders have also been studied in transgenic monkeys [191]. Due to the highest similarity with humans, monkeys are preferred for understanding the pathways involved in the progression of PD [191], microcephaly [192], AD [193], and sleep disorders [194].
The brain neurons lack the potential of regeneration; hence, the aging degeneration leads to severe consequences of brain dysfunctions. The neurodegenerative diseases/disorders are characterized by slow progression at an early stage. These diseases affect elderly persons especially in developed countries where the life expectancy is high [195]. These diseases include Parkinson’s Disease (PD), Progressive Supra-nuclear Palsy (PSP), Multi-System Atrophy (MSA), Alzheimer's Disease (AD), Fronto-Temporal Dementia (FTD), and Dementia with Lewy Bodies (DLB). PD is a progressive neurodegenerative disorder that causes slowness of movement and rigidity in the body. It is characterized by neuronal loss in the substantia nigra and the other brain regions. It is associated with the formation of intracellular protein inclusions known as Lewy bodies (LBs) in the neurons [196, 197].
Nanotechnology has provided a platform for the transfer of drugs across the BBB. Researchers are trying to build liposomes, loaded with nanoparticles to gain access through the BBB [198, 199]. More research is required to determine effective strategies for the improvement of patients with brain disorders. Delivering drugs across the BBB is one of the most promising application of nanotechnology in the field of clinical neuroscience. Nanoparticles may potentially carry out multiple tasks in a pre-defined sequence, which may be important for the delivery of drugs across the BBB [200-204].
CONCLUSION
Due to the involvement of non-genetic factors in the progression of human brain disorders, the research has been focused more on the study of epigenetic factors. In this context the data available from the GWAS and the databases developed for neurodegenerative diseases have proven a great boon in the area. Although there are several models to study the neurodegenerative disease but still there is a need for other specialized techniques, especially for neuropsychiatric disorders due to the overlapping of symptoms. BD gene has attempted not only to address the genetic complexities of bipolar disorders but also overlapping symptoms of both schizophrenia and Major Depressive Disorders (MDD).
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


