Insights into Super-host Plant Species of Galling Insects in the Neotropical Region
Abstract
Background:
The term ‘super host’ plant is often used in the literature surrounding plant-galling interactions, but the different contexts in which the term is used generates doubt and confusion due to the absence of a systematic definition of the term’s meaning.
Objective:
In this study, we used 60 well-defined plant-galling assemblages to propose a systematic definition of super-host plants at the local and regional level. In addition, we investigated factors that explain the number of galling species per host plant at different geographic scales.
Methods:
Plant-galling assemblages were compiled from an extensive literature review on insect gall inventories carried out in Brazil.
Results:
We found 888 host plant species belonging to 94 families and 340 genera hosting 2,376 insect gall morphotypes. At a local scale, 33.2% of host plant species harbored one insect gall morphotype and 12.2% hosted two gall morphotypes, making up 45.4% of the host plant species in each locality. At the regional scale, 51.5% of host plant species harbored one insect gall morphotype, and 17.9% of host plant species hosted two gall morphotypes, corresponding to 69.4% of all host plant species. Based on the average number of galling species per plant species, we classified the plant species into: 1) Host species; 2) Multi-host species and 3) Super-host species. The super-host plant species that showed the greatest richness of gall morphotypes at the local level were Baccharis reticularia and Adenocalymma neoflavidu. Furthermore, we found a positive relationship between plant life-form architectural complexity and the number of galling species at the local level. At the regional scale, we registered five super-host species (Guapira opposita, Protium heptaphyllum, Copaifera langsdorffii, Myrcia splendens, and Byrsonima sericea) which hosted 21 or more insect gall morphotypes. The number of galling species per host plant species at the regional scale was influenced positively by geographic distribution rank and number of biomes in which each species of the plant occurs.
Conclusion:
The present study stands out as the first of its kind to provide a systematic standardization for the super-host plants and to investigate factors influencing these species.
1. INTRODUCTION
Interactions involving host plants and galling insects are among the most diverse and specialized in nature [1, 2]. In the Neotropical region, a great diversity of plant-galling interactions has been studied in recent years [3-5]. Estimates indicate that more than 15,400 species of galling insects and more than 29,000 species of host plants exist in the neotropics [3]. Galling species tend to be species-specific with their hosts [6], but host plant species can host a variable number of galling insect species [7]. In most cases, the number of galling insect species per host plant species is usually one or two, although some host species can house more than 20 different galling species [8, 9].
In studies investigating plant-galling interactions in the Neotropical region, the term ‘super-host’ is frequently used to designate plant species that host a high richness of galling species [10]. For example, Costa et al. [11] defined Copaifera langsdorffii (Fabaceae) as a super-host because it houses 23 galling species. In a separate study, this same host species was recorded as a super-host while hosting 17 galling species [12]. Copaifera oblongifolia, another congeneric species was also named a super-host by Fagundes et al. [13] and Coutinho et al. [14], hosting 15 galling species. Goetz et al. [15] named Piper aduncum (Piperaceae) and Mikania glomerata (Asteraceae) super-hosts, harboring eight and six galling species, respectively. In another study, Maia and Oliveira [16] used the term super-host to describe Guapira opposita (Nyctaginaceae) which hosted four galling insect species. In an extreme case, Ribeiro et al. [17] defined Moquiniastrum pulchrum (Asteraceae) as a super-host plant, by housing two galling species. These examples illustrate how the term ‘super-host’ is frequently used in galling insect studies, often in an indiscriminate and non-standardized way.
The number of herbivorous insects hosted by host plants can be influenced by several factors at different scales [18]. At the local scale, studies have pointed to plant traits such as life-form as influencing the distribution of galling species, with trees usually hosting greater galling insect diversity than non-arboreal plants [19]. These results agree with the hypothesis that host plants with higher architectural complexity house more insect herbivore species [20, 21]. At the regional scale, the plant’s geographic range has been hypothesized as an important predictor of the diversity of herbivorous insects hosted by a plant species [18]. This pattern can be explained by the fact that hosts with a wide distribution have contact with a greater diversity of herbivores, which promotes the accumulation of more interactions throughout their distribution [22]. The aforementioned factors (amongst others), may potentially explain the occurrence of super-host plant species, though this remains a knowledge gap of plant-galling interactions.
In this context, the standardized definition of the term ‘super-host’ and the investigation of factors influencing the occurrence of these species are important to guide studies of insect gall diversity in neotropical environments. In this study, we propose a definition to differentiate super-host plants at the local and regional level using a dataset compiled from the literature surrounding plant-galling inventories in Brazil. We aim to answer the following questions: 1) What defines super-host plants at the local level? 2) Do super-host plants vary locally? 3) Are there factors that explain the occurrence of super-host taxa at the local level? 4) What defines super-host plants at the regional level? 5) Are there factors that explain the occurrence of super-host taxa at the regional level?
2. METHODS
2.1. Data Compilation
We carried out a comprehensive literature search for studies reporting assemblages of gall insects associated with their host plant in the SciVerse Scopus, Portal Capes, and Google Scholar databases (until 2017), using the following combinations of keywords: (plant*) and (galls*) and (interaction* or web*) and (survey* or list*). It is important to note that we compiled only inventories of the richness of host plants and galling insects, and we did not consider literature focused on a single species of plant or galling insect, to ensure that all studies included had searched for all galls occurring in the community. Thus, only those studies with plant-gall insect assemblages that met all of the following criteria were included in our study: 1) At least 3 host plant species; 2) At least 3 host plants hosting a minimum of 3 galling insect species (i.e., insect gall morphotypes); and 3) An indication that all plants could potentially be utilized by the gall insect assemblages in the list (i.e., no spatial mismatch). Overall, 60 local plant-galling assemblages were selected from 31 studies (Supplementary Material 1). The latitudinal distribution of the plant-galling assemblages ranged from 1°20’ S to 29°28’ S, and their altitudes ranged from 10 to 1,860 m.a.s.l.
2.2. Plant Species
For each local plant-galling assemblage we checked the synonymy of host plant using "The Plant List" database (www. theplantlist.org). We also used additional information, such as the Tropicos (www.tropicos.org) databases to refine a list of each family, genus, and host plant species where they were sampled. Host plants that were taxonomically determined only at the genus level (i.e., species identified as “sp.”) were also added to the list.
2.2.1. Local and Regional Gall Morphospecies Diversity
We used compiled data to calculate the richness of insect galls at local and regional levels. At the local level, we considered the number of gall morphotypes described in each host plant species within the community. For the categorization of the morphotypes, we considered the original morphological (i.e., plant organ, shape, color, and pilosity) description made by the authors of the studies. We did not consider the size of galls as an important character in our study due to the high variability in this characteristic. The use of morphotypes as a surrogate for the species of galling insects is widespread in the literature due to the high specificity of galls and the lack of taxonomic studies of gallers [5]. At the regional level, we considered the sum of unique morphotypes on each host plant species in the different plant-galling communities.
It is important to emphasize that our measure of gall richness at the regional level, is strongly dependent on sampling effort for each host plant species because plant species that have occurred in several communities are more likely to accumulate more galls over space [5]. However, we believe that as we adopt the criterion of compiling only studies that inventoried the entire plant community, this effect can be mitigated. Besides, the authors did not look a priori at specific plant species, they collected information for all plants in the community. Thus, it is expected that the plant species that are registered in different studies are species with wide distribution between habitats and localities.
2.3. Data Analyses
At the regional level, we used a Generalized Linear Model (GLM) to investigate plant-related factors (life-forms, endemism, geographic range, biome occurrence, vegetation occurrence) on the galling species richness. Predictive variables were ordered as ordinal (sequential) variables. For the plant life-form architectural complexity, we used the following categories: 1 = herb, 2 = liana, 3 = shrub and 4 = tree. With regards to endemism, we considered plant species with occurrence only in Brazil as endemic and species occurring in Brazil and other countries as non-endemic. The categories used for the geographical distribution area were: 1 = distribution restricted to a small area/locality, 2 = biome (in a single state), 3 = biome (occurring in several states), 4 = Brazil (occurring in different biomes in the same state) and 5 = Brazil (occurring in different biomes and states). For vegetation, we used the following categories: 1 = grassland, 2 = savanna and 3 = forest. We used this ranking because there is an increasing gradient of structural complexity (in terms of biomass, tree cover, and height of vegetation) grassland <savanna <forest. We also accounted for the number of biomes within which each plant species occurs. At the local level, we also used a GLM to investigate whether galling species richness is influenced by plant life-form using the same life-form categories as above. All of the models were built using the error of Poisson distribution. All statistical analyses were performed in R version 3.4.1 [23].
3. RESULTS
A total of 888 host plant species belonging to 94 families and 340 genera were analyzed hosting 2,376 insect gall morphotypes. The most important host plant family was Fabaceae, hosting 291 (12.2%) insect gall morphotypes, followed by Asteraceae (263; 11.0%), Myrtaceae (260; 10.9%), Melastomataceae (126; 5.3%), Malpighiaceae (111; 4.6%), and Burseraceae (100; 4.2%). The most important of host plant genera were Myrcia (Myrtaceae) with 106 (4.4%), Protium (Burseraceae) with 85 (3.6%), Eugenia (Myrtaceae) with 84 (3.5%), Baccharis (Asteraceae) with 81 (3.4%), and Byrsonima (Malpighiaceae) with 78 (3.2%) insect gall morphotypes.
At a local scale, 33.2 ± 10.5% of host plant species in each locality harbored one insect gall morphotype and 12.2 ± 4.8% hosted two gall morphotypes, totaling 45.4% of the host plant species (Fig. 1; Table 1). Considering all host plant species, the mean number of gall morphotypes per plant species was 2.67 ± 3.33. We considered plant-species with more than double of this mean (i.e., six or more species) as super host plants at a local scale (Table 2), which correspond to less than 1% of host plant species in each locality. For a given locality, the maximum number of insect gall morphotypes per plant species ranged between 3 and 10. Plant species that hosted between three and six insect gall morphotypes were categorized as multi-host plant species (Table 2). Super-host plants at the local level were represented by 23 species occurring across 24 localities (Table 3). The species that hosted the most gall morphotypes at individual sites were Baccharis reticularia with 10 insect gall morphotypes in the Parque Estadual do Itacolomi and Adenocalymma neoflavidum (Bignoniaceae) with nine gall morphotypes in the Floresta Nacional Saracá-Taquera. Host plant species more frequently listed in inventories of galling insects were Guapira opposite (eight studies), Eremanthus erythropappus (Asteraceae) (three studies) and Protium heptaphyllum (three studies) (Table 3). We found a positive relationship between the life-form and the number of galling species at the local level (Deviance = 14.46, df = 1779, p < 0.001).
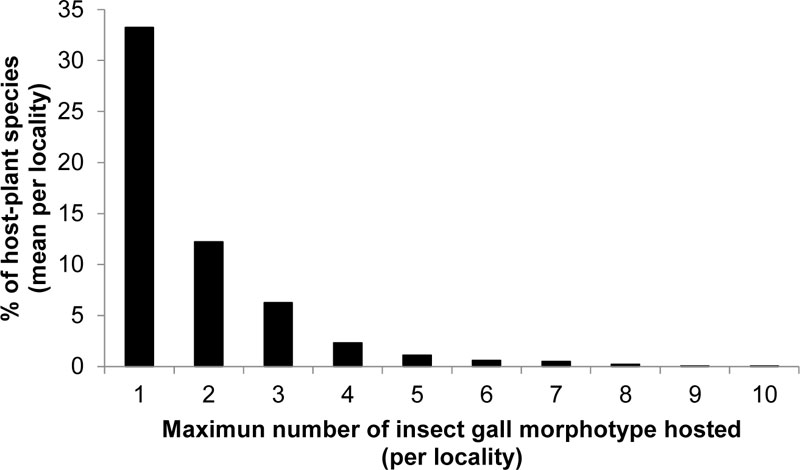
| Range (Gall Morphotypes per Plant Species) | Percent of Host Plant Species per Locality | |||
|---|---|---|---|---|
| Mean | SD | Max | Min | |
| 1 | 33.2 | 10.6 | 63.6 | 12.5 |
| 2 | 12.2 | 4.8 | 21.3 | 0 |
| 3 | 6.2 | 3.2 | 17.1 | 0 |
| 4 | 2.3 | 2.1 | 8.3 | 0 |
| 5 | 1.1 | 1.6 | 5.7 | 0 |
| 6 | 0.6 | 1.1 | 4.2 | 0 |
| 7 | 0.5 | 1.2 | 6.3 | 0 |
| 8 | 0.2 | 0.6 | 2.9 | 0 |
| 9 | 0.0 | 0.2 | 1.6 | 0 |
| 10 | 0.0 | 0.2 | 1.8 | 0 |
| Ecological Scale | Range of Richness of Insect Gall Morphotypes | Category of Host Plant Species |
|---|---|---|
| Local scale | 1 to 3 | Host plant species |
| 3 to 6 | Multi-host plant species | |
| 6 or more | Super-host plant species | |
| Regional scale | 1 to 3 | Host plant species |
| 6 to 20 | Multi-host plant species | |
| 21 or more species | Super-host plant species |
| Super-host Plant Species | Plant Family | Number of Insect Gall Morphotypes | Locality | References |
|---|---|---|---|---|
| Baccharis pseudomyriocephala | Asteraceae | 10 | Parque Estadual do Itacolomi | Carneiro et al. [24] |
| Adenocalymma neoflavidum | Bignoniaceae | 9 | Floresta Nacional Saracá-Taquera | Araújo et al. [25] |
| Baccharis platypoda | Asteraceae | 8 | Parque Estadual do Brigadeiro | Coelho et al. [26] |
| Protium heptaphyllum | Burseraceae | 8 | Serra de São José Cachoeira do Mangue | Maia and Fernandes [27] |
| Guapira opposita | Nyctaginaceae | 8 | Bertioga | Maia [28] |
| Mikania biformis | Asteraceae | 8 | Bertioga | Maia [28] |
| Guapira opposita | Nyctaginaceae | 8 | Maricá | Maia [28] |
| Copaifera langsdorfii | Fabaceae | 8 | Cachoeira da Lua | Maia [29] |
| Guapira opposita | Nyctaginaceae | 8 | Ilha da Marambaia - Armação | Rodrigues et al. [30] |
| Baccharis reticularia | Asteraceae | 7 | Parque Estadual do Itacolomi | Carneiro et al. [24] |
| Baccharis platypoda | Asteraceae | 7 | RPPN do Caraça | Carneiro et al. [24] |
| Baccharis reticularia | Asteraceae | 7 | RPPN do Caraça | Carneiro et al. [24] |
| Byrsonima coccolobifolia | Malpighiaceae | 7 | RPPN do Caraça | Carneiro et al. [24] |
| Protium sagotianum | Burseraceae | 7 | Platô Bacaba - Porto de Trombetas | Maia [31] |
| Myrcia splendens | Myrtaceae | 7 | Bertioga | Maia [28] |
| Ocotea pulchella | Lauraceae | 7 | Bertioga | Maia [28] |
| Eugenia astringens | Myrtaceae | 7 | Jurubatiba | Maia [28] |
| Guapira opposita | Nyctaginaceae | 7 | Jurubatiba | Maia [28] |
| Mikania biformis | Asteraceae | 7 | Guaratuba | Maia et al. [32] |
| Myrcia splendens | Myrtaceae | 7 | Praia do Itaguaré | Maia et al. [32] |
| Guapira opposita | Nyctaginaceae | 7 | Estação Biológica de Santa Lúcia | Maia et al. [33] |
| Mikania laevigata | Asteraceae | 7 | Parque Natural Municipal São Lourenço | Maia et al. [33] |
| Protium heptaphyllum | Burseraceae | 7 | Mata Semicaudicifolia - UFG | Silva et al. [34] |
| Styrax pohlii | Styracaceae | 7 | Mata Semicaudicifolia - UFG | Silva et al. [34] |
| Guapira opposita | Nyctaginaceae | 7 | Ilha da Marambaia - Sitio | Rodrigues et al. [30] |
| Eremanthus erythropappus | Asteraceae | 6 | Parque Estadual do Itacolomi | Carneiro et al. [24] |
| Byrsonima coccolobifolia | Malpighiaceae | 6 | Parque Estadual do Biribiri | Carneiro et al. [24] |
| Eremanthus erythropappus | Asteraceae | 6 | RPPN do Caraça | Carneiro et al. [24] |
| Eremanthus erythropappus | Asteraceae | 6 | Serra do Ouro Branco | Carneiro et al. [24] |
| Copaifera langsdorffii | Fabaceae | 6 | Campus Pampulha - UFMG | Fernandes et al. [35] |
| Copaifera langsdorffii | Fabaceae | 6 | Serra de São José Cachoeira do Mangue | Maia and Fernandes [27] |
| Myrcia multiflora | Myrtaceae | 6 | Restinga de Carapebus | Maia [36] |
| Miconia stenostachya | Melastomataceae | 6 | Platô Bacaba - Porto de Trombetas | Maia [31] |
| Tetragastris panamensis | Burseraceae | 6 | Platô Bacaba - Porto de Trombetas | Maia [31] |
| Myrcia sylvatica | Myrtaceae | 6 | Cachoeira da Lua | Maia [29] |
| Eugenia astringens | Myrtaceae | 6 | Carapebus | Maia [29] |
| Myrcia multiflora | Myrtaceae | 6 | Carapebus | Maia [29] |
| Calophyllum brasiliense | Calophyllaceae | 6 | Vale das Borboletas | Maia [29] |
| Guapira opposita | Nyctaginaceae | 6 | Praia do Itaguaré | Maia et al. [32] |
| Psychotria vellosiana | Rubiaceae | 6 | Estação Biológica de Santa Lúcia | Maia et al. [33] |
| Guapira opposita | Nyctaginaceae | 6 | Parque Natural Municipal São Lourenço | Maia et al. [33] |
| Protium heptaphyllum | Burseraceae | 6 | Reserva Ecologica de Saltinho | Santos et al. [37] |
| Super-Host Plant Species | Plant Family | Number of Insect Gall Morphotypes | References |
|---|---|---|---|
| Guapira opposita | Nyctaginaceae | 40 | Bregonci et al. [38], Maia [36], Rodrigues et al. [30], Maia et al. [32], Santos et al. [37], Maia and Carvalho-Fernandez [39], Maia and Silva [40], Maia [28], Maia et al. [33]. |
| Protium heptaphyllum | Burseraceae | 35 | Maia [36], Maia and Fernandes [27], Santos et al. [37], Silva et al. [34], Fernandes et al. [41], Maia and Carvalho-Fernandes [39], Maia [29], Santana and Isaias [42]. |
| Copaifera langsdorffii | Fabaceae | 27 | Maia [29], Santos et al. [43], Fernandes et al. [35], Urso-Guimarães and Scareli-Santos [44], Coelho et al. [45], Fernandes et al. [46], Maia and Fernandes [27], Silva et al. [34], Luz et al. [47], Santana and Isaias [42]. |
| Myrcia splendens | Myrtaceae | 23 | Rodrigues et al. [30], Coelho et al. [45], Fernandes et al. [46], Maia et al. [32], Maia [29], Santana and Isaias [42]. |
| Byrsonima sericea | Malpighiaceae | 22 | Bregonci et al. [38], Maia [36], Oliveira and Maia [48], Rodrigues et al. [30], Santos et al. [37], Maia and Carvalho-Fernandes [39], Maia and Silva [40], Maia [28]. |
At the regional scale, 51.5% of host plant species harbored one insect gall morphotype, and 17.9% of host plant species hosted two gall morphotypes, corresponding to 69.4% of all host plant species in this study. We considered plant-species with more than double the mean of insect gall morphotypes per host plant species (2.67 ± 3.33) as multi-host plants (i.e., host plants with six or more insect gall morphotypes) (Table 2), which correspond to 91 host plant species with a mean of 10.20 ± 5.82 gall morphotypes. Plant species with more than double the mean number of galls of multi-host plants (i.e., 21 or more insect gall morphotypes) were considered as super-host plants (Table 2). This classification resulted in a total of five super-host plant-species at the regional level: Guapira opposita (40 insect gall morphotypes), Protium heptaphyllum (35), Copaifera langsdorffii (27), Myrcia splendens (23), and Byrsonima sericea (22) (Table 4). Super-host plant species had a mean of 29.4 ± 7.82 insect gall morphotypes. The number of galling insect species per host plant species at the regional scale was influenced positively by life-form (Deviance = 5.13, df = 845, p = 0.02), geographic distribution rank (Deviance = 68.93, df = 841, p < 0.001), number of biomes (Deviation = 74.41, df = 840, p < 0.001) and by increased vegetation complexity (Deviation = 87.14, df = 823, p < 0.001).
4. DISCUSSION
In this study, we adopted the reasoning that super-host species are those that have a greater variety of insect galls compared to the average galling species richness per plant species occurring within the community. The criterion adopted in our study indicates that super-hosts at a local level are the species with six or more galling species, which represent twice the average diversity of galling species per plant species. Our criterion at the regional level was more rigorous because on a macroscale all plant species accumulate more species of herbivorous insects than on a local scale [18]. Thus, we refer to the plant species that at the regional level have an intermediate species richness of insect galls as multi-hosts and we consider super-hosts to be only those species that have more than twice the average galling species richness of multi-hosts. Thus, at the regional level, we considered super-host plants to be the only species that hosted 21 or more species of galling insects. Based on the criteria presented here, our study represents the first systematized definition of super-host plants both at the community (local scale) and the macrogeographic level (regional scale).
Our approach revealed, from a wide range of data, that the species of super-host plants can vary greatly over different geographic scales. Although 23 species can be considered super-hosts at the local level (i.e., had six species of galling insects or more), we observed that some species are frequently listed as super-hosts such as Guapira opposita. We also found that at the local scale plants with a high diversity of insect galls tend to be more complex, that is, they have arboreal life-form [19]. The architectural complexity of trees tends to be higher compared to other plant life-forms because trees have higher height, the number of shoots, branches, and leaves with the crown volume [20]. In addition, the trees are larger and have greater biomass than other life-forms which allows a greater accumulation of herbivorous insects [19]. These different parameters positively influence the richness of insect galls as they represent a greater availability of resources (i.e., biomass) and oviposition sites (i.e., types of tissues) for galling insects [11, 19]. At the regional level, super-host plant (i.e., had 21 species of galling insects or more) were represented by only five plant species, with Guapira opposita and Protium heptaphyllum that hosted the highest numbers of insect galls. Our results show that, in general, super-host plants on a macrogeographic scale are those with a greater range of distribution and that occur in a greater number of biomes.
The plant Guapira opposita was also the super-host species at the local level most frequently recorded in our database, being recorded eight times. This species is a tree widely distributed across South America, and in Brazil is registered in Amazonia, Caatinga, Cerrado, and Atlantic Forest [49]. With regards to vegetation types, Guapira opposita is registered in riparian forest, seasonal semideciduous forest, rainforest, mixed rainforest, restinga, and vegetation on rocky outcrops [49]. In Brazil, this species has been registered as a super-host in areas of restinga and coastal vegetation [28, 30, 33]. The compiled database showed that this super-host plant presents an elevated alpha diversity of galling insects across several Brazilian locations. This can be explained by their structural architecture, given that trees tend to host a greater diversity of herbivorous insects compared to other plant life-forms [20]. Besides, we hypothesized that the great diversity of galling insects associated with Guapira opposita is related to the adaptive irradiation of gall-midges (Cecidomyiidae, Diptera) on this plant. For example, there is evidence of the occurrence of many species of Bruggmania associated with this host [50], which indicates that processes of sympatric speciation happened during the evolutionary history of this system.
Other important super-host plant species at the local scale were Eremanthus erythropappus and Protium heptaphyllum, which were each recorded three times. Eremanthus erythropappus is an endemic tree in Brazil that occurs in Cerrado and Atlantic Forest, and being frequently recorded in rocky savanna, typical savanna, riparian forest, and seasonal semideciduous forest [51]. This species has been recorded as a super-host in different areas of the Espinhaço Range [24]. Protium heptaphyllum is a neotropical tree registered in Brazil, in the Amazonia, Caatinga, Cerrado, and Atlantic Forest. Among the types of vegetation that Protium heptaphyllum occurs are campinarana, riparian forest, terra firme forest, rainforest, restinga, and savannah [52]. This species has been registered as a super-host in the Atlantic Forest [37], Semidecidual forest [34], and Cerrado [27].
At the regional level, Guapira opposita with 40 insect gall morphotypes and Protium heptaphyllum with 35, were the most important super-host plants. As previously indicated, these plant species were also the most recurrent super-hosts at the local level. This result indicates that in addition to a high alpha diversity locally, these super-host species also have a high beta diversity, due to the replacement of galling species throughout space [53]. Our results also indicate that breadth of geographic distribution and number of biomes positively influenced the number of galling insect species per host plant species at the regional scale. In a broad review, Nyman [22] argues that hosts with a wide range of distribution tend to accumulate more species of herbivorous insects as a result of added possibilities for local adaptation. The high numbers of galling species hosted by Guapira opposita and Protium heptaphyllum can be explained by the wide-ranging distribution of these species across the Neotropical region [49, 52], given that the two species occur across different countries in Latin America, and are registered in several biomes in Brazil.
CONCLUSION
Based on our initial questions and results, we conclude that in tropical and sub-tropical Brazil: 1) Super-host plants at the local level are species that host six or more galling species; 2) Species of super-host plants vary widely between communities, but some species are recurrently registered as super-hosts, such as Guapira opposita; 3) Plant life-form influences the richness of insect galls that they host, with super-hosts tending to be arboreal; 4) On a regional scale, super-host plants are those species that host 21 or more galling insect species; 5) Species of super-host plants at the regional level tend to have a wider range of geographic distribution and occur across several different biomes. The present study stands out as the first to provide a systematic standardization for super-host plants and to investigate factors influencing these species.
AUTHORS' CONTRIBUTIONS
JMGR and WSA designed the experiment. JMGR and CGHP performed the data compilation. WSA performed statistical analyses. All authors prepared the manuscript.
ETHICAL APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author [W.S.A] on request.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors thank to Jean Carlos Santos for the suggestions to the manuscript, and to Instituto de Investigaciones de la Amazonia Peruana and Universidade Estadual de Montes Claros for logistical support. We also thank Emma Docherty for the English review.


